Ann Surg Treat Res.
2018 Feb;94(2):57-62. 10.4174/astr.2018.94.2.57.
The effect of mesenchymal stem cell use on intra-abdominal adhesions in a rat model
- Affiliations
-
- 1Department of General Surgery, Faculty of Medicine, Kırıkkale University, Kırıkkale, Turkey. gokhankaracaa@yahoo.com
- 2Department of Biochemistry, Istanbul University, Istanbul, Turkey.
- 3Department of General Surgery, Hacettepe University, School of Medicine, Ankara, Turkey.
- 4Department of General Surgery, Alaca State Hospital, Çorum, Turkey.
- 5Department of Biochemistry, Bezmialem Vakif University Medical Faculty, Istanbul, Turkey.
- KMID: 2402846
- DOI: http://doi.org/10.4174/astr.2018.94.2.57
Abstract
- PURPOSE
Intra-abdominal adhesions (IAA) are among the most frequently seen pathologies in general surgery practice with an increased morbidity and mortality. In the present study, we investigated the effect of locally applied mesenchymal stem cells (MSCs) on IAA.
METHODS
Twenty-four Wistar Albino rats were used in the study. The rats were divided into three groups including: Sham, control, and MSCs group. On day 0, cecum was reached under anesthesia in all groups, except the Sham group. Scraping with a sponge was performed until petechial bleeding occurred. The control group received no treatment. In the stem cell group, MSCs were applied topically immediately after surgery on adhesions. The rats were sacrificed on day 10 and colon tissues and blood samples were collected for macroscopic, histopathological, and biochemical analysis.
RESULTS
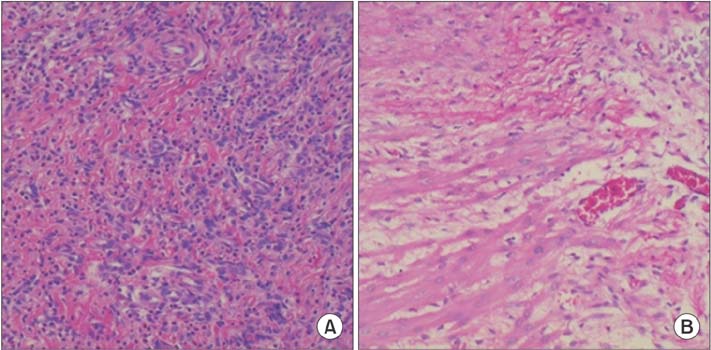
In our study, E-selectin, P-selectin, TNF-α and IL-1 levels were statistically significantly lower in the MSC group than the control group, while the sham group has the lowest levels. In both the macroscopic and histopathological analyses (Zühlke's scale), the least amount of adhesion was observed in the Sham group. In addition, although there was less adhesion in the MSC group than the control group, the difference did not reach statistical significance.
CONCLUSION
Topical MSC application immediately after surgery suppresses the inflammatory process. However it was found to be ineffective in histopathological and macroscopic examinations performed on the 10th day.
MeSH Terms
Figure
Reference
-
1. Drollette CM, Badawy SZ. Pathophysiology of pelvic adhesions. Modern trends in preventing infertility. J Reprod Med. 1992; 37:107–121.2. diZerega GS. Contemporary adhesion prevention. Fertil Steril. 1994; 61:219–235.
Article3. diZerega GS, Campeau JD. Peritoneal repair and post-surgical adhesion formation. Hum Reprod Update. 2001; 7:547–555.
Article4. Brunschwig A, Robbins GF. Regeneration of peritoneum: experimental observations and clinical experience in radical resections of intra-abdominal cancer. In : XVth Congress of the Society of International Chirurgie; Lisbonne. Bruxelles: Henri de Smedt. 1953. p. 756–765.5. Johnson FR, Whıttıng HW. Repair of parietal peritoneum. Br J Surg. 1962; 49:653–660.
Article6. Ellıs H, Harrıson W, Hugh TB. The healıng of perıtneum under normal and pathologıcal condıtıons. Br J Surg. 1965; 52:471–476.7. Lucas PA, Warejcka DJ, Young HE, Lee BY. Formation of abdominal adhesions is inhibited by antibodies to transforming growth factor-beta1. J Surg Res. 1996; 65:135–138.8. Lucas PA, Warejcka DJ, Zhang LM, Newman WH, Young HE. Effect of rat mesenchymal stem cells on development of abdominal adhesions after surgery. J Surg Res. 1996; 62:229–232.
Article9. Wang N, Li Q, Zhang L, Lin H, Hu J, Li D, et al. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS One. 2012; 7:e43768.
Article10. Wang N, Shao Y, Mei Y, Zhang L, Li Q, Li D, et al. Novel mechanism for mesenchymal stem cells in attenuating peritoneal adhesion: accumulating in the lung and secreting tumor necrosis factor α-stimulating gene-6. Stem Cell Res Ther. 2012; 3:51.
Article11. Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000; 100:157–168.12. Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005; 2:8.13. da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008; 26:2287–2299.
Article14. Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, et al. Comparison of immunomodulatory properties of mesen chymal stem cells derived from adult human tissues. Cell Immunol. 2009; 259:150–156.15. Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for atte nua tion of scar formation during wound healing. Stem Cell Res Ther. 2012; 3:20.16. Elkins TE, Bury RJ, Ritter JL, Ling FW, Ahokas RA, Homsey CA, et al. Adhesion pre ven tion by solutions of sodium carboxy methylcellulose in the rat. I. Fertil Steril. 1984; 41:926–928.17. Niyaz M, Gurpınar OA, Gunaydın S, Onur MA. Isolation, culturing and characterization of rat adipose tissue derived mesenchymal stem cells: a simple technique. Turk J Biol. 2012; 36:658–664.18. Nair SK, Bhat IK, Aurora AL. Role of proteolytic enzyme in the prevention of postoperative intraperitoneal adhesions. Arch Surg. 1974; 108:849–853.
Article19. Zuhlke HV, Lorenz EM, Straub EM, Savvas V. Pathophysiology and classification of adhesions. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1990; 1009–1016.20. Müller SA, Treutner KH, Haase G, Kinzel S, Tietze L, Schumpelick V. Effect of intraperitoneal antiadhesive fluids in a rat peri tonitis model. Arch Surg. 2003; 138:286–290.21. Tarhan OR, Barut I, Sutcu R, Akdeniz Y, Akturk O. Pentoxifylline, a methyl xanthine derivative, reduces peritoneal ad hesions and increases peritoneal fibrinolysis in rats. Tohoku J Exp Med. 2006; 209:249–255.22. Aysan E, Basak F, Kinaci E, Yanar H, Coskun H. Experimental adhesion model: effect of viscosities of fluids put in the peri toneal cavity on preventing peritoneal adhesions. Exp Anim. 2007; 56:349–354.23. Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003; 9:263–268.
Article24. Gao Y, Li N, Fei R, Chen Z, Zheng S, Zeng X. P-Selectin-mediated acute inflammation can be blocked by chemically modified heparin, RO-heparin. Mol Cells. 2005; 19:350–355.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mesenchymal Stem Cell Therapy for Intrac table Neonatal Disorders
- Combination Cell Therapy with Mesenchymal Stem Cells and Neural Stem Cells for Brain Stroke in Rats
- L-Theanine-Treated Adipose-Derived Mesenchymal Stem Cells Alleviate the Cytotoxicity Induced by N-Nitrosodiethylamine in Liver
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease
- Human Liver Stem Cell Transplantation Alleviates Liver Fibrosis in a Rat Model of CCl 4 -Induced Liver Fibrosis