Intest Res.
2018 Jan;16(1):83-89. 10.5217/ir.2018.16.1.83.
Efficacy and safety of the adalimumab biosimilar Exemptia as induction therapy in moderate-to-severe ulcerative colitis
- Affiliations
-
- 1Department of Internal Medicine, Dayanand Medical College & Hospital, Ludhiana, India.
- 2Department of Gastroenterology, Dayanand Medical College & Hospital, Ludhiana, India. ajitsood10@gmail.com
- 3Department of Pathology, Dayanand Medical College & Hospital, Ludhiana, India.
- 4Department of Pharmacology, Dayanand Medical College & Hospital, Ludhiana, India.
- KMID: 2402651
- DOI: http://doi.org/10.5217/ir.2018.16.1.83
Abstract
- BACKGROUND/AIMS
Data on the efficacy and safety of the adalimumab biosimilar Exemptia are limited.
METHODS
Patients with moderate-to-severe active steroid-refractory ulcerative colitis (UC) treated at Dayanand Medical College and Hospital, India were offered cyclosporine A, biologicals or biosimilars, or surgery. A retrospective analysis was conducted on patients who were treated with the adalimumab biosimilar, Exemptia. These patients were administered an induction dosing schedule of 160 mg Exemptia at week 0, 80 mg at week 2, and then 40 mg every other week from week 4 to 8. The clinical response and remission were assessed at week 8 using Mayo score.
RESULTS
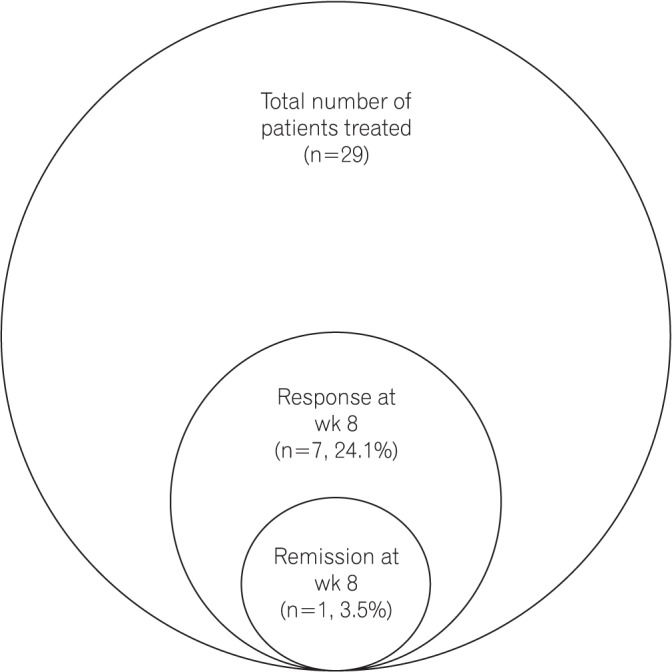
A total of 29 patients (62.1% male; mean age, 34.9 ± 9.7 years) with moderate-to-severe steroid-refractory active UC (mean disease duration, 6.3±5.1 years; pancolitis in 9 patients [31.1%]; left-sided colitis in 20 patients [68.9%]) were treated with the Exemptia induction dosing schedule. The mean Mayo score at presentation was 8.2±1.4. At week 8, clinical response was observed in 7 patients (24.1%), whereas clinical remission was observed only in 1 patient (3.5%). Among the non-responders (n=21), 4 patients required colectomy, 1 died, 1 was lost to follow-up, 10 were offered fecal microbiota transplant, 3 were administered infliximab, and 2 patients were administered cyclosporine and tacrolimus, respectively. Four patients (13.8%) developed extrapulmonary tuberculosis.
CONCLUSIONS
The adalimumab biosimilar Exemptia has limited efficacy for the attainment of clinical response and remission in moderate-to-severe steroid-refractory UC, with a significant risk of acquisition or reactivation of tuberculosis in developing countries such as India.
MeSH Terms
-
Adalimumab*
Appointments and Schedules
Biosimilar Pharmaceuticals
Colectomy
Colitis
Colitis, Ulcerative*
Cyclosporine
Developing Countries
Humans
India
Infliximab
Lost to Follow-Up
Male
Microbiota
Retrospective Studies
Tacrolimus
Tuberculosis
Ulcer*
Adalimumab
Biosimilar Pharmaceuticals
Cyclosporine
Infliximab
Tacrolimus
Figure
Reference
-
1. Rubin DT, Siegel CA, Kane SV, et al. Impact of ulcerative colitis from patients' and physicians'perspectives: results from the UC. NORMAL survey. Inflamm Bowel Dis. 2009; 15:581–588. PMID: 19067414.2. Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996; 38:905–910. PMID: 8984031.
Article3. Danese S, Angelucci E, Malesci A, Caprilli R. Biological agents for ulcerative colitis: hypes and hopes. Med Res Rev. 2008; 28:201–218. PMID: 17464967.
Article4. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476. PMID: 16339095.
Article5. Peyrin-Biroulet L, Laclotte C, Roblin X, Bigard MA. Adalimumab induction therapy for ulcerative colitis with intolerance or lost response to infliximab: an open-label study. World J Gastroenterol. 2007; 13:2328–2332. PMID: 17511032.
Article6. Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011; 60:780–787. PMID: 21209123.
Article7. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012; 142:257–265.e3. PMID: 22062358.
Article8. Humira (adalimumab). Food and Drug Administration. April 23, 2017. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/125057orig1s232ltr.pdf.9. Oussalah A, Laclotte C, Chevaux JB, et al. Long-term outcome of adalimumab therapy for ulcerative colitis with intolerance or lost response to infliximab: a single-centre experience. Aliment Pharmacol Ther. 2008; 28:966–972. PMID: 18652603.
Article10. Afif W, Leighton JA, Hanauer SB, et al. Open-label study of adalimumab in patients with ulcerative colitis including those with prior loss of response or intolerance to infliximab. Inflamm Bowel Dis. 2009; 15:1302–1307. PMID: 19408340.
Article11. Trinder MW, Lawrance IC. Efficacy of adalimumab for the management of inflammatory bowel disease in the clinical setting. J Gastroenterol Hepatol. 2009; 24:1252–1257. PMID: 19220669.
Article12. García-Bosch O, Gisbert JP, Cañas-Ventura A, et al. Observational study on the efficacy of adalimumab for the treatment of ulcerative colitis and predictors of outcome. J Crohns Colitis. 2013; 7:717–722. PMID: 23142005.
Article13. Gies N, Kroeker KI, Wong K, Fedorak RN. Treatment of ulcerative colitis with adalimumab or infliximab: long-term follow-up of a single-centre cohort. Aliment Pharmacol Ther. 2010; 32:522–528. PMID: 20500733.
Article14. Taxonera C, Estellés J, Fernández-Blanco I, et al. Adalimumab induction and maintenance therapy for patients with ulcerative colitis previously treated with infliximab. Aliment Pharmacol Ther. 2011; 33:340–348. PMID: 21133961.
Article15. Armuzzi A, Biancone L, Daperno M, et al. P230 Adalimumab in active ulcerative colitis: a “real-life” observational study. J Crohns Colitis. 2012; 6(Suppl 1):S101–S101.
Article16. Ferrante M, Karmiris K, Compernolle G, Ballet V, Vermeire S, Noman M. Efficacy of adalimumab in patients with ulcerative colitis: restoration of serum levels after dose escalation results in a better long-term outcome. Gut. 2011; 60(Suppl 3):A72.17. Guidelines on evaluation of similar biotherapeutic products (SBPs). World Health Organization;Accessed May 4, 2017. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf.18. Park SH, Kim YH, Lee JH, et al. Post-marketing study of biosimilar infliximab (CT-P13) to evaluate its safety and efficacy in Korea. Expert Rev Gastroenterol Hepatol. 2015; 9(Suppl 1):35–44. PMID: 26395533.
Article19. Jung YS, Park DI, Kim YH, et al. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol. 2015; 30:1705–1712. PMID: 25974251.
Article20. Kang YS, Moon HH, Lee SE, Lim YJ, Kang HW. Clinical experience of the use of CT-P13, a biosimilar to infliximab in patients with inflammatory bowel disease: a case series. Dig Dis Sci. 2015; 60:951–956. PMID: 25326115.
Article21. Gecse KB, Lovász BD, Farkas K, et al. Efficacy and safety of the biosimilar infliximab CT-P13 treatment in inflammatory bowel diseases: a prospective, multicentre, nationwide cohort. J Crohns Colitis. 2016; 10:133–140. PMID: 26661272.
Article22. Farkas K, Rutka M, Bálint A, et al. Efficacy of the new infliximab biosimilar CT-P13 induction therapy in Crohn's disease and ulcerative colitis: experiences from a single center. Expert Opin Biol Ther. 2015; 15:1257–1262. PMID: 26134250.
Article23. Park DI. Current status of biosimilars in the treatment of inflammatory bowel diseases. Intest Res. 2016; 14:15–20. PMID: 26884730.
Article24. Adalimumab biosimilar. autoimmune disorders. rheumatoid arthritis. juvenile idiopathic arthritis. psoriatic arthritis, ankylosing spondylitis. Exemptia;Accessed May 4, 2017. https://exemptia.com/zydus-launches-worlds-first-biosimilar-of-adalimumab-2/.25. Jani RH, Gupta R, Bhatia G, et al. A prospective, randomized, double-blind, multicentre, parallel-group, active controlled study to compare efficacy and safety of biosimilar adalimumab (Exemptia; ZRC-3197) and adalimumab (Humira) in patients with rheumatoid arthritis. Int J Rheum Dis. 2016; 19:1157–1168. PMID: 26176644.
Article26. Sood A, Midha V, Sood N, et al. Cyclosporine in the treatment of severe steroid refractory ulcerative colitis: a retrospective analy-sis of 24 cases. Indian J Gastroenterol. 2008; 27:232–235. PMID: 19405256.27. Sood A, Midha V, Sharma S, et al. Infliximab in patients with severe steroid-refractory ulcerative colitis: Indian experience. Indian J Gastroenterol. 2014; 33:31–34. PMID: 23999683.
Article28. TB statistics for India: national and state statistics. TB Facts.org;April 26, 2017. http://www.tbfacts.org/tb-statistics-india/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Golimumab Therapy in Ulcerative Colitis
- Understanding the Role of Adalimumab in the Treatment of Moderately to Severely Active Ulcerative Colitis
- Efficacy and Safety of Adalimumab in Moderately to Severely Active Cases of Ulcerative Colitis: A Meta-Analysis of Published Placebo-Controlled Trials
- Reversible Corpus Callosal Lesions Associated with the Use of Adalimumab for Ulcerative Colitis
- Treatment of Recalcitrant Pyoderma Gangrenosum with Ulcerative Colitis by Adalimumab Injection