Intest Res.
2018 Jan;16(1):75-82. 10.5217/ir.2018.16.1.75.
Efficacy of restarting anti-tumor necrosis factor α agents after surgery in patients with Crohn's disease
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan. sakikoh@cc.okayama-u.ac.jp
- 2Department of Gastroenterological Surgery, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan.
- 3Second Department of Internal Medicine, Wakayama Medical University, Wakayama, Japan.
- KMID: 2402650
- DOI: http://doi.org/10.5217/ir.2018.16.1.75
Abstract
- BACKGROUND/AIMS
The efficacy of anti-tumor necrosis factor α (anti-TNFα) antibodies for postoperative Crohn's disease (CD) in patients who were treated with these agents prior to surgery is largely unknown.
METHODS
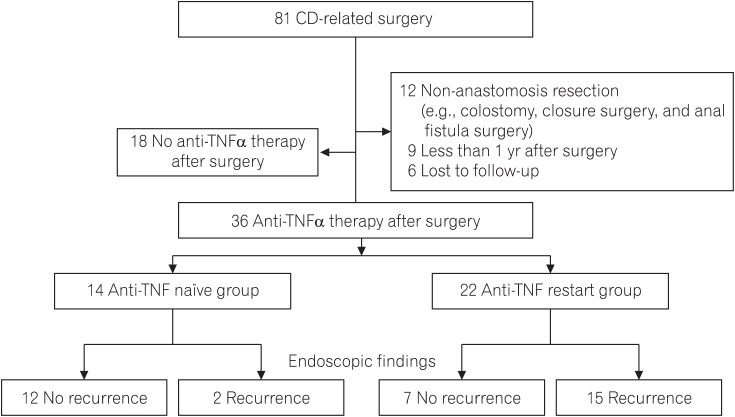
CD patients who underwent intestinal resection and received anti-TNFα agents after surgery were divided into 2 groups according to the presence or absence of preoperative anti-TNFα treatment: anti-TNFα restart group or anti-TNFα naïve group. Endoscopic recurrence after surgery was examined according to the preoperative conditions, including administration of anti-TNFα agents before surgery.
RESULTS
Thirty-six patients received anti-TNFα antibody after surgery: 22 in the anti-TNFα restart group and 14 in the anti-TNFα naïve group. Endoscopic recurrence after surgery was more frequently observed in the anti-TNFα restart group than in the anti-TNFα naïve group (68% vs. 14%, P < 0.001). Multivariate analysis revealed the following significant risk factors of endoscopic recurrence after surgery: anti-TNF restart group (odds ratio [OR], 28.10; 95% CI, 3.08-722.00), age at diagnosis < 23 years (OR, 24.30; 95% CI, 1.67-1,312.00), serum albumin concentration at surgery < 3.3 g/dL (OR, 34.10; 95% CI, 1.72-2,804.00), and presence of inflammation outside of the surgical site (OR, 21.40; 95% CI, 1.02-2,150.00). Treatment intensification for patients with endoscopic recurrence in the anti-TNFα restart group showed limited responses, with only 1 of 12 patients achieving endoscopic remission.
CONCLUSIONS
The efficacy of restarting anti-TNFα antibody treatment after surgery was limited, and treatment intensification or a change to different classes of biologics should be considered for those patients.
MeSH Terms
Figure
Cited by 2 articles
-
The old versus the new: which do you keep in postoperative Crohn's disease?
Paulo Gustavo Kotze, Christopher Ma, Miguel Regueiro, Remo Panaccione
Intest Res. 2018;16(2):319-320. doi: 10.5217/ir.2018.16.2.319.Author's Reply
Sakiko Hiraoka, Jun Kato, Hiroyuki Okada
Intest Res. 2018;16(2):321-322. doi: 10.5217/ir.2018.16.2.321.
Reference
-
1. Steinhart AH, Girgrah N, McLeod RS. Reliability of a Crohn's disease clinical classification scheme based on disease behavior. Inflamm Bowel Dis. 1998; 4:228–234. PMID: 9741028.
Article2. Rutgeerts P. Review article: recurrence of Crohn's disease after surgery-the need for treatment of new lesions. Aliment Pharmacol Ther. 2006; 24(Suppl 3):29–32. PMID: 16961741.
Article3. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010; 139:1147–1155. PMID: 20637205.
Article4. Frolkis AD, Lipton DS, Fiest KM, et al. Cumulative incidence of second intestinal resection in Crohn's disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol. 2014; 109:1739–1748. PMID: 25331349.
Article5. Kotze PG, Saad-Hossne R, Spinelli A. Endoscopic postoperative recurrence rates in Crohn's disease in Korea: the beginning of a new approach? Intest Res. 2014; 12:258–259. PMID: 25349602.
Article6. Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV Jr. Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn's disease after surgery: a systematic review and network meta-analysis. Gastroenterology. 2015; 148:64–76.e2. PMID: 25263803.
Article7. Kotze PG, Spinelli A, da Silva RN, et al. Conventional versus biological therapy for prevention of postoperative endoscopic recurrence in patients with Crohn's disease: an international, multicenter, and observational study. Intest Res. 2015; 13:259–265. PMID: 26131001.
Article8. Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011; 60:571–607. PMID: 21464096.
Article9. Lichtenstein GR, Hanauer SB, Sandborn WJ. Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2009; 104:465–483. PMID: 19174807.
Article10. Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010; 4:28–62. PMID: 21122489.
Article11. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990; 99:956–963. PMID: 2394349.
Article12. Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn's disease. Am J Gastroenterol. 2008; 103:944–948. PMID: 18028512.
Article13. Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimizing response to anti-TNF therapy in Crohn's disease: algorithm for practical management. Aliment Pharmacol Ther. 2016; 43:30–51. PMID: 26515897.
Article14. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010; 4:355–366. PMID: 21122530.
Article15. Amiot A, Setakhr V, Seksik P, et al. Long-term outcome of enterocutaneous fistula in patients with Crohn's disease treated with anti-TNF therapy: a cohort study from the GETAID. Am J Gastroenterol. 2014; 109:1443–1449. PMID: 25091063.
Article16. Yaari S, Benson A, Aviran E, et al. Factors associated with surgery in patients with intra-abdominal fistulizing Crohn's disease. World J Gastroenterol. 2016; 22:10380–10387. PMID: 28058018.
Article17. Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014; 20:2247–2259. PMID: 25358062.
Article18. Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn's disease. Gastroenterology. 2006; 130:650–656. PMID: 16530505.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preoperative use of anti-tumor necrosis factor therapy in Crohn's disease: promises and pitfalls
- Biological Therapy for Inflammatory Bowel Disease in Children
- Recurrence of Tuberculosis after Resuming a TNF-Inhibitor in a Patient with Crohn's Disease
- Anti-tumor Necrosis Factor Therapy for Crohn Disease: Friend or Foe to the Surgeon?
- Old and New Biologics and Small Molecules in Inflammatory Bowel Disease: Anti-Tumor Necrosis Factors