Clin Exp Vaccine Res.
2018 Jan;7(1):1-15. 10.7774/cevr.2018.7.1.1.
Challenges of influenza A viruses in humans and animals and current animal vaccines as an effective control measure
- Affiliations
-
- 1College of Veterinary Medicine, Konkuk University, Seoul, Korea. lyoo@konkuk.ac.kr
- KMID: 2402533
- DOI: http://doi.org/10.7774/cevr.2018.7.1.1
Abstract
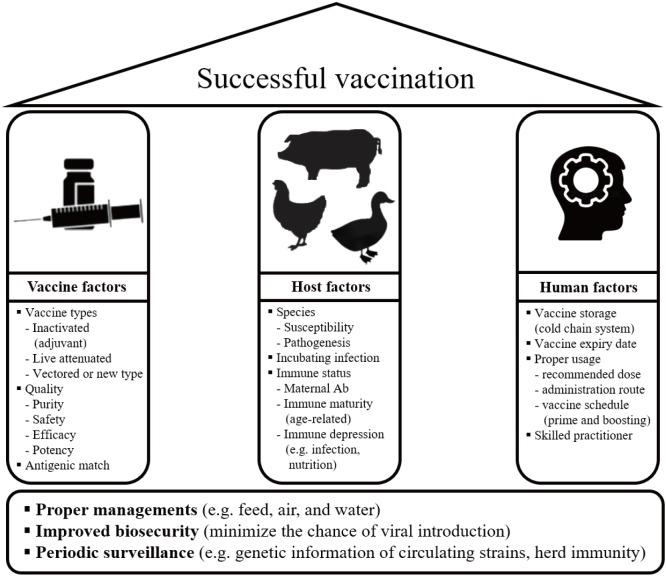
- Influenza A viruses (IAVs) are genetically diverse and variable pathogens that share various hosts including human, swine, and domestic poultry. Interspecies and intercontinental viral spreads make the ecology of IAV more complex. Beside endemic IAV infections, human has been exposed to pandemic and zoonotic threats from avian and swine influenza viruses. Animal health also has been threatened by high pathogenic avian influenza viruses (in domestic poultry) and reverse zoonosis (in swine). Considering its dynamic interplay between species, prevention and control against IAV should be conducted effectively in both humans and animal sectors. Vaccination is one of the most efficient tools against IAV. Numerous vaccines against animal IAVs have been developed by a variety of vaccine technologies and some of them are currently commercially available. We summarize several challenges in control of IAVs faced by human and animals and discuss IAV vaccines for animal use with those application in susceptible populations.
Keyword
MeSH Terms
Figure
Reference
-
1. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56:152–179. PMID: 1579108.
Article2. Webster RG, Govorkova EA. Continuing challenges in influenza. Ann N Y Acad Sci. 2014; 1323:115–139. PMID: 24891213.
Article3. Cauldwell AV, Long JS, Moncorge O, Barclay WS. Viral determinants of influenza A virus host range. J Gen Virol. 2014; 95:1193–1210. PMID: 24584475.
Article4. Short KR, Richard M, Verhagen JH, et al. One health, multiple challenges: the inter-species transmission of influenza A virus. One Health. 2015; 1:1–13. PMID: 26309905.
Article5. Zhou L, Ren R, Yang L, et al. Sudden increase in human infection with avian influenza A(H7N9) virus in China, September-December 2016. Western Pac Surveill Response J. 2017; 8:6–14. PMID: 28409054.
Article6. Iuliano AD, Jang Y, Jones J, et al. Increase in human infections with avian influenza A(H7N9) virus during the fifth epidemic, October 2016-February 2017. MMWR Morb Mortal Wkly Rep. 2017; 66:254–255. PMID: 28278147.7. Smith GJ, Vijaykrishna D, Bahl J, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009; 459:1122–1125. PMID: 19516283.
Article8. Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009; 325:197–201. PMID: 19465683.9. Vincent A, Awada L, Brown I, et al. Review of influenza A virus in swine worldwide: a call for increased surveillance and research. Zoonoses Public Health. 2014; 61:4–17. PMID: 23556412.
Article10. Peiris M, Yen HL. Animal and human influenzas. Rev Sci Tech. 2014; 33:539–553. PMID: 25707182.
Article11. Hayward AC, Fragaszy EB, Bermingham A, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014; 2:445–454. PMID: 24717637.
Article12. Horby PW. Community studies of influenza: new knowledge, new questions. Lancet Respir Med. 2014; 2:430–431. PMID: 24717636.
Article13. World Organisation for Animal Health. Terrestrial Animal Health Code [Internet]. Paris: World Organisation for Animal Health;2017. cited 2017 Oct 28. Available from: http://www.oie.int/international-standard-setting/terrestrial-code/access-online.14. Shen YY, Ke CW, Li Q, et al. Novel reassortant avian influenza A(H5N6) viruses in humans, Guangdong, China, 2015. Emerg Infect Dis. 2016; 22:1507–1509. PMID: 27331418.
Article15. Nguyen DC, Uyeki TM, Jadhao S, et al. Isolation and characterization of avian influenza viruses, including highly pathogenic H5N1, from poultry in live bird markets in Hanoi, Vietnam, in 2001. J Virol. 2005; 79:4201–4212. PMID: 15767421.
Article16. Lee CW, Senne DA, Linares JA, et al. Characterization of recent H5 subtype avian influenza viruses from US poultry. Avian Pathol. 2004; 33:288–297. PMID: 15223555.
Article17. Lee DH, Bertran K, Kwon JH, Swayne DE. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J Vet Sci. 2017; 18:269–280. PMID: 28859267.
Article18. Lee DH, Torchetti MK, Winker K, Ip HS, Song CS, Swayne DE. Intercontinental spread of Asian-origin H5N8 to North America through beringia by migratory birds. J Virol. 2015; 89:6521–6524. PMID: 25855748.
Article19. Anderson T, Capua I, Dauphin G, et al. FAO-OIE-WHO Joint Technical Consultation on avian influenza at the human-animal interface. Influenza Other Respir Viruses. 2010; 4(Suppl 1):1–29.20. Pfeiffer DU, Otte MJ, Roland-Holst D, Zilberman D. A one health perspective on HPAI H5N1 in the Greater Mekong sub-region. Comp Immunol Microbiol Infect Dis. 2013; 36:309–319. PMID: 23260375.
Article21. World Health Organization. Fact sheets 211 influenza (seasonal) [Internet]. Geneva: World Health Organization;2016. cited 2017 Oct 15. Available from: http://www.who.int/mediacentre/factsheets/fs211/en.22. Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007; 25:5086–5096. PMID: 17544181.
Article23. World Health Organization. Influenza update [Internet]. Geneva: World Health Organization;2017. cited 2017 Oct 15. Available from: http://www.who.int/influenza/surveillance_monitoring/updates/latest_update_GIP_surveillance/en.24. Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006; 12:15–22. PMID: 16494711.
Article25. Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. Characterization of the 1918 influenza virus polymerase genes. Nature. 2005; 437:889–893. PMID: 16208372.
Article26. Anhlan D, Grundmann N, Makalowski W, Ludwig S, Scholtissek C. Origin of the 1918 pandemic H1N1 influenza A virus as studied by codon usage patterns and phylogenetic analysis. RNA. 2011; 17:64–73. PMID: 21068184.
Article27. Reid AH, Fanning TG, Janczewski TA, Lourens RM, Taubenberger JK. Novel origin of the 1918 pandemic influenza virus nucleoprotein gene. J Virol. 2004; 78:12462–12470. PMID: 15507633.
Article28. Gibbs MJ, Gibbs AJ. Molecular virology: was the 1918 pandemic caused by a bird flu? Nature. 2006; 440:E8. PMID: 16641948.29. Fanning TG, Slemons RD, Reid AH, Janczewski TA, Dean J, Taubenberger JK. 1917 avian influenza virus sequences suggest that the 1918 pandemic virus did not acquire its hemagglutinin directly from birds. J Virol. 2002; 76:7860–7862. PMID: 12097598.
Article30. Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe. 2010; 7:440–451. PMID: 20542248.
Article31. Gu M, Xu L, Wang X, Liu X. Current situation of H9N2 subtype avian influenza in China. Vet Res. 2017; 48:49. PMID: 28915920.
Article32. Epperson S, Jhung M, Richards S, et al. Human infections with influenza A(H3N2) variant virus in the United States, 2011-2012. Clin Infect Dis. 2013; 57(Suppl 1):S4–S11. PMID: 23794729.
Article33. Bowman AS, Walia RR, Nolting JM, et al. Influenza A (H3N2) virus in swine at agricultural fairs and transmission to humans, Michigan and Ohio, USA, 2016. Emerg Infect Dis. 2017; 23:1551–1555. PMID: 28820376.34. World Health Organization. Influenza: monthly risk assessment summary (7 December 2017) [Internet]. Geneva: World Health Organization;2017. cited 2017 Dec 7. Available from: http://www.who.int/influenza/human_animal_interface/HAI_Risk_Assessment/en.35. Krauss S, Stallknecht DE, Slemons RD, et al. The enigma of the apparent disappearance of Eurasian highly pathogenic H5 clade 2.3.4.4 influenza A viruses in North American waterfowl. Proc Natl Acad Sci U S A. 2016; 113:9033–9038. PMID: 27457948.
Article36. Ellis TM, Bousfield RB, Bissett LA, et al. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 2004; 33:492–505. PMID: 15545029.
Article37. Desvaux S, Marx N, Ong S, et al. Highly pathogenic avian influenza virus (H5N1) outbreak in captive wild birds and cats, Cambodia. Emerg Infect Dis. 2009; 15:475–478. PMID: 19239769.
Article38. Harfoot R, Webby RJ. H5 influenza, a global update. J Microbiol. 2017; 55:196–203. PMID: 28243942.
Article39. World Health Organization. H5N1 avian influenza: timeline of major events [Internet]. Geneva: World Health Organization;2014. cited 2017 Nov 14. Available from: http://www.who.int/influenza/human_animal_interface/en.40. World Organisation for Animal Health. Economic analysis: prevention versus outbreak costs [Internet]. Paris: World Organisation for Animal Health;2007. cited 2017 Oct 28. Available from: http://www.oie.int/fileadmin/Home/eng/Support_to_OIE_Members/docs/pdf/OIE_-_Cost-Benefit_Analysis__Part_I_.pdf.41. U.S. Government Accountability Office. Avian influenza: USDA has taken actions to reduce risks but needs a plan to evaluate its efforts [Internet]. Washington, DC: U.S. Government Accountability Office;2017. cited 2017 Nov 4. Available from: http://www.gao.gov/assets/690/684086.pdf.42. Hyundai Research Institute. Spread of AIV at utmost speed ever and the resulting economic losses [Internet]. Seoul: Hyundai Research Institute;2016. [cited 2017 Nov 1. Available from: http://hri.co.kr/upload/publication/2016121494516[1].pdf.43. Rushton J, Gilbert W. The economics of animal health: direct and indirect costs of animal disease outbreaks [Internet]. Paris: World Organisation for Animal Health;2016. cited 2017 Nov 4. Available from: https://www.oie.int/eng/session2016/sg84/02-Monday/Rushton_Technical_Paper_TT1_final_revision.pdf.44. Swayne DE, Hill RE, Clifford J. Safe application of regionalization for trade in poultry and poultry products during highly pathogenic avian influenza outbreaks in the USA. Avian Pathol. 2017; 46:125–130. PMID: 27817200.
Article45. Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW. Diseases of swine. 10th ed. Chichester: Wiley-Blackwell;2012.46. Smith RD, Keogh-Brown MR. Macroeconomic impact of pandemic influenza and associated policies in Thailand, South Africa and Uganda. Influenza Other Respir Viruses. 2013; 7(Suppl 2):64–71. PMID: 24034487.
Article47. Ito T, Couceiro JN, Kelm S, et al. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol. 1998; 72:7367–7373. PMID: 9696833.
Article48. Trebbien R, Larsen LE, Viuff BM. Distribution of sialic acid receptors and influenza A virus of avian and swine origin in experimentally infected pigs. Virol J. 2011; 8:434. PMID: 21902821.
Article49. Chan RW, Karamanska R, Van Poucke S, et al. Infection of swine ex vivo tissues with avian viruses including H7N9 and correlation with glycomic analysis. Influenza Other Respir Viruses. 2013; 7:1269–1282. PMID: 24001121.50. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood FS, Jain S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009; 360:2605–2615. PMID: 19423869.
Article51. Rajao DS, Walia RR, Campbell B, et al. Reassortment between Swine H3N2 and 2009 pandemic H1N1 in the United States resulted in influenza A viruses with diverse genetic constellations with variable virulence in pigs. J Virol. 2017; 91:e01763–e01716. PMID: 27928015.
Article52. Nelson MI, Gramer MR, Vincent AL, Holmes EC. Global transmission of influenza viruses from humans to swine. J Gen Virol. 2012; 93:2195–2203. PMID: 22791604.
Article53. Shortridge KF, Webster RG, Butterfield WK, Campbell CH. Persistence of Hong Kong influenza virus variants in pigs. Science. 1977; 196:1454–1455. PMID: 867041.
Article54. Gilbert M, Conchedda G, Van Boeckel TP, et al. Income disparities and the global distribution of intensively farmed chicken and pigs. PLoS One. 2015; 10:e0133381. PMID: 26230336.
Article55. Swayne DE, Kapczynski DR. Vaccines, vaccination, and immunology for avian influenza viruses in poultry. In : Swayne DE, editor. Avian influenza. Ames, IW: Blackwell Publishing;2008. p. 407–451.56. Vincent AL, Perez DR, Rajao D, et al. Influenza A virus vaccines for swine. Vet Microbiol. 2017; 206:35–44. PMID: 27923501.
Article57. Peiris JS, Cowling BJ, Wu JT, et al. Interventions to reduce zoonotic and pandemic risks from avian influenza in Asia. Lancet Infect Dis. 2016; 16:252–258. PMID: 26654122.
Article58. Bowman AS, Nolting JM, Nelson SW, Slemons RD. Subclinical influenza virus A infections in pigs exhibited at agricultural fairs, Ohio, USA, 2009-2011. Emerg Infect Dis. 2012; 18:1945–1950. PMID: 23171654.
Article59. Swayne DE. Impact of vaccines and vaccination on global control of avian influenza. Avian Dis. 2012; 56(4 Suppl):818–828. PMID: 23402099.
Article60. Swayne DE, Pavade G, Hamilton K, Vallat B, Miyagishima K. Assessment of national strategies for control of high-pathogenicity avian influenza and low-pathogenicity notifiable avian influenza in poultry, with emphasis on vaccines and vaccination. Rev Sci Tech. 2011; 30:839–870. PMID: 22435196.
Article61. Lee DH, Song CS. H9N2 avian influenza virus in Korea: evolution and vaccination. Clin Exp Vaccine Res. 2013; 2:26–33. PMID: 23596587.
Article62. Perk S, Golender N, Banet-Noach C, et al. Phylogenetic analysis of hemagglutinin, neuraminidase, and nucleoprotein genes of H9N2 avian influenza viruses isolated in Israel during the 2000-2005 epizootic. Comp Immunol Microbiol Infect Dis. 2009; 32:221–238. PMID: 18249445.
Article63. Naeem K, Siddique N. Use of strategic vaccination for the control of avian influenza in Pakistan. Dev Biol (Basel). 2006; 124:145–150. PMID: 16447505.64. Lewis NS, Russell CA, Langat P, et al. The global antigenic diversity of swine influenza A viruses. Elife. 2016; 5:e12217. PMID: 27113719.
Article65. Kong W, Ye J, Guan S, Liu J, Pu J. Epidemic status of Swine influenza virus in china. Indian J Microbiol. 2014; 54:3–11. PMID: 24426160.
Article66. Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. Swine influenza viruses a North American perspective. Adv Virus Res. 2008; 72:127–154. PMID: 19081490.67. Rashid A, Rasheed K, Akhtar M. Factors influencing vaccine efficacy: a general review. J Anim Plant Sci. 2009; 19:22–25.68. Swayne DE. Animal influenza. 2nd ed. Ames, IW: John Wiley and Sons Inc;2016.69. Rahn J, Hoffmann D, Harder TC, Beer M. Vaccines against influenza A viruses in poultry and swine: status and future developments. Vaccine. 2015; 33:2414–2424. PMID: 25835575.
Article70. Spackman E, Pantin-Jackwood MJ. Practical aspects of vaccination of poultry against avian influenza virus. Vet J. 2014; 202:408–415. PMID: 25296849.
Article71. Trombetta CM, Perini D, Mather S, Temperton N, Montomoli E. Overview of serological techniques for influenza vaccine evaluation: past, present and future. Vaccines (Basel). 2014; 2:707–734. PMID: 26344888.
Article72. Park AW, Daly JM, Lewis NS, Smith DJ, Wood JL, Grenfell BT. Quantifying the impact of immune escape on transmission dynamics of influenza. Science. 2009; 326:726–728. PMID: 19900931.
Article73. Abdelwhab EM, Hassan MK, Abdel-Moneim AS, et al. Introduction and enzootic of A/H5N1 in Egypt: virus evolution, pathogenicity and vaccine efficacy ten years on. Infect Genet Evol. 2016; 40:80–90. PMID: 26917362.
Article74. Lee DH, Fusaro A, Song CS, Suarez DL, Swayne DE. Poultry vaccination directed evolution of H9N2 low pathogenicity avian influenza viruses in Korea. Virology. 2016; 488:225–231. PMID: 26655240.
Article75. Lee CW, Senne DA, Suarez DL. Effect of vaccine use in the evolution of Mexican lineage H5N2 avian influenza virus. J Virol. 2004; 78:8372–8381. PMID: 15254209.
Article76. Vijaykrishna D, Smith GJ, Pybus OG, et al. Long-term evolution and transmission dynamics of swine influenza A virus. Nature. 2011; 473:519–522. PMID: 21614079.
Article77. Harder TC, Grosse Beilage E, Lange E, et al. Expanded cocirculation of stable subtypes, emerging lineages, and new sporadic reassortants of porcine influenza viruses in swine populations in Northwest Germany. J Virol. 2013; 87:10460–10476. PMID: 23824819.
Article78. Cecchinato M, Catelli E, Lupini C, et al. Avian metapneumovirus (AMPV) attachment protein involvement in probable virus evolution concurrent with mass live vaccine introduction. Vet Microbiol. 2010; 146:24–34. PMID: 20447777.
Article79. Franzo G, Tucciarone CM, Cecchinato M, Drigo M. Porcine circovirus type 2 (PCV2) evolution before and after the vaccination introduction: a large scale epidemiological study. Sci Rep. 2016; 6:39458. PMID: 27991573.
Article80. Kwon T, Lee DU, Yoo SJ, Je SH, Shin JY, Lyoo YS. Genotypic diversity of porcine circovirus type 2 (PCV2) and genotype shift to PCV2d in Korean pig population. Virus Res. 2017; 228:24–29. PMID: 27867029.
Article81. Yoo SJ, Kwon T, Kang K, et al. Genetic evolution of classical swine fever virus under immune environments conditioned by genotype 1-based modified live virus vaccine. Transbound Emerg Dis. 2018; 1. 10. [Epub ahead of print]. DOI: 10.1111/tbed.12798.
Article82. Rodpothong P, Auewarakul P. Viral evolution and transmission effectiveness. World J Virol. 2012; 1:131–134. PMID: 24175217.
Article83. Rajao DS, Loving CL, Gauger PC, Kitikoon P, Vincent AL. Influenza A virus hemagglutinin protein subunit vaccine elicits vaccine-associated enhanced respiratory disease in pigs. Vaccine. 2014; 32:5170–5176. PMID: 25077416.
Article84. Gauger PC, Vincent AL, Loving CL, et al. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine. 2011; 29:2712–2719. PMID: 21310191.85. Vincent AL, Lager KM, Janke BH, Gramer MR, Richt JA. Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine. Vet Microbiol. 2008; 126:310–323. PMID: 17719188.
Article86. Khurana S, Loving CL, Manischewitz J, et al. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med. 2013; 5:200ra114.
Article87. Sun S, Cui Z, Wang J, Wang Z. Protective efficacy of vaccination against highly pathogenic avian influenza is dramatically suppressed by early infection of chickens with reticuloendotheliosis virus. Avian Pathol. 2009; 38:31–34. PMID: 19145513.
Article88. Rajao DS, Sandbulte MR, Gauger PC, et al. Heterologous challenge in the presence of maternally-derived antibodies results in vaccine-associated enhanced respiratory disease in weaned piglets. Virology. 2016; 491:79–88. PMID: 26874588.
Article89. Centers for Disease Control and Prevention. Principles of vaccination [Internet]. Atlanta, GA: Centers for Disease Control and Prevention;2015. cited 2017 Nov 4. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/prinvac.html.90. Dutta A, Huang CT, Lin CY, et al. Sterilizing immunity to influenza virus infection requires local antigen-specific T cell response in the lungs. Sci Rep. 2016; 6:32973. PMID: 27596047.
Article91. Savill NJ, St Rose SG, Keeling MJ, Woolhouse ME. Silent spread of H5N1 in vaccinated poultry. Nature. 2006; 442:757. PMID: 16915278.
Article92. Poetri ON, Van Boven M, Claassen I, et al. Silent spread of highly pathogenic Avian Influenza H5N1 virus amongst vaccinated commercial layers. Res Vet Sci. 2014; 97:637–641. PMID: 25301756.
Article93. Wright PF. Vaccine preparedness: are we ready for the next influenza pandemic? N Engl J Med. 2008; 358:2540–2543. PMID: 18550873.94. Zhou F, Zhou J, Ma L, et al. High-yield production of a stable Vero cell-based vaccine candidate against the highly pathogenic avian influenza virus H5N1. Biochem Biophys Res Commun. 2012; 421:850–854. PMID: 22554519.
Article95. Hu AY, Tseng YF, Weng TC, et al. Production of inactivated influenza H5N1 vaccines from MDCK cells in serum-free medium. PLoS One. 2011; 6:e14578. PMID: 21283675.
Article96. Ping J, Lopes TJ, Nidom CA, et al. Development of high-yield influenza A virus vaccine viruses. Nat Commun. 2015; 6:8148. PMID: 26334134.
Article97. Centers for Disease Control and Prevention. Cell-based flu vaccines [Internet]. Atlanta, GA: Centers for Disease Control and Prevention;2016. cited 2017 Nov 4. Available from: https://www.cdc.gov/flu/protect/vaccine/cell-based.htm.98. Smith JH, Papania M, Knaus D, et al. Nebulized live-attenuated influenza vaccine provides protection in ferrets at a reduced dose. Vaccine. 2012; 30:3026–3033. PMID: 22075083.
Article99. Liu Q, Mena I, Ma J, et al. Newcastle disease virus-vectored H7 and H5 live vaccines protect chickens from challenge with H7N9 or H5N1 avian influenza viruses. J Virol. 2015; 89:7401–7408. PMID: 25926639.
Article100. Ma J, Lee J, Liu H, et al. Newcastle disease virus-based H5 influenza vaccine protects chickens from lethal challenge with a highly pathogenic H5N2 avian influenza virus. NPJ Vaccines. 2017; 2:33. PMID: 29263888.
Article101. Hu Z, Liu X, Jiao X, Liu X. Newcastle disease virus (NDV) recombinant expressing the hemagglutinin of H7N9 avian influenza virus protects chickens against NDV and highly pathogenic avian influenza A (H7N9) virus challenges. Vaccine. 2017; 35(48 Pt B):6585–6590. PMID: 29042201.
Article102. Nakaya T, Cros J, Park MS, et al. Recombinant Newcastle disease virus as a vaccine vector. J Virol. 2001; 75:11868–11873. PMID: 11689668.
Article103. Ma W, Richt JA. Swine influenza vaccines: current status and future perspectives. Anim Health Res Rev. 2010; 11:81–96. PMID: 20462470.
Article104. Suarez DL, Pantin-Jackwood MJ. Recombinant viral-vectored vaccines for the control of avian influenza in poultry. Vet Microbiol. 2017; 206:144–151. PMID: 27916319.
Article105. Tsunekuni R, Hikono H, Tanikawa T, Kurata R, Nakaya T, Saito T. Recombinant avian paramyxovirus serotypes 2, 6, and 10 as vaccine vectors for highly pathogenic avian influenza in chickens with antibodies against Newcastle disease virus. Avian Dis. 2017; 61:296–306. PMID: 28957006.
Article106. Yoshida A, Samal SK. Avian Paramyxovirus Type-3 as a Vaccine vector: identification of a genome location for high level expression of a foreign gene. Front Microbiol. 2017; 8:693. PMID: 28473820.
Article107. Bublot M, Pritchard N, Swayne DE, et al. Development and use of fowlpox vectored vaccines for avian influenza. Ann N Y Acad Sci. 2006; 1081:193–201. PMID: 17135511.
Article108. Richard-Mazet A, Goutebroze S, Le Gros FX, Swayne DE, Bublot M. Immunogenicity and efficacy of fowlpox-vectored and inactivated avian influenza vaccines alone or in a prime-boost schedule in chickens with maternal antibodies. Vet Res. 2014; 45:107. PMID: 25359591.
Article109. Swayne DE. Avian influenza vaccines and therapies for poultry. Comp Immunol Microbiol Infect Dis. 2009; 32:351–363. PMID: 18442853.
Article110. Iavarone C, O'Hagan DT, Yu D, Delahaye NF, Ulmer JB. Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines. 2017; 16:871–881. PMID: 28701102.
Article111. Diken M, Kreiter S, Selmi A, et al. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 2011; 18:702–708. PMID: 21368901.
Article112. Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011; 29:185–214. PMID: 21219183.
Article113. Moon SL, Wilusz J. In vitro transcription of modified RNAs. Methods Mol Biol. 2012; 941:171–180. PMID: 23065561.
Article114. Kariko K, Muramatsu H, Ludwig J, Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011; 39:e142. PMID: 21890902.115. Jones KL, Drane D, Gowans EJ. Long-term storage of DNA-free RNA for use in vaccine studies. Biotechniques. 2007; 43:675–681. PMID: 18072597.
Article116. Hoerr I, Obst R, Rammensee HG, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol. 2000; 30:1–7. PMID: 10602021.117. Paul S, Stang A, Lennartz K, Tenbusch M, Uberla K. Selection of a T7 promoter mutant with enhanced in vitro activity by a novel multi-copy bead display approach for in vitro evolution. Nucleic Acids Res. 2013; 41:e29. PMID: 23074193.
Article118. Petsch B, Schnee M, Vogel AB, et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol. 2012; 30:1210–1216. PMID: 23159882.
Article119. Bahl K, Senn JJ, Yuzhakov O, et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther. 2017; 25:1316–1327. PMID: 28457665.
Article120. Ekiert DC, Friesen RH, Bhabha G, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011; 333:843–850. PMID: 21737702.
Article121. Wiersma LC, Rimmelzwaan GF, de Vries RD. Developing universal influenza vaccines: hitting the nail, not just on the head. Vaccines (Basel). 2015; 3:239–262. PMID: 26343187.
Article122. Nayak B, Kumar S, DiNapoli JM, et al. Contributions of the avian influenza virus HA, NA, and M2 surface proteins to the induction of neutralizing antibodies and protective immunity. J Virol. 2010; 84:2408–2420. PMID: 20032181.
Article123. Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev. 2013; 26:476–492. PMID: 23824369.
Article124. Heinen PP, Rijsewijk FA, de Boer-Luijtze EA, Bianchi AT. Vaccination of pigs with a DNA construct expressing an influenza virus M2-nucleoprotein fusion protein exacerbates disease after challenge with influenza A virus. J Gen Virol. 2002; 83:1851–1859. PMID: 12124449.
Article125. Tumpey TM, Alvarez R, Swayne DE, Suarez DL. Diagnostic approach for differentiating infected from vaccinated poultry on the basis of antibodies to NS1, the nonstructural protein of influenza A virus. J Clin Microbiol. 2005; 43:676–683. PMID: 15695663.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influenza Vaccines: Unmet Needs and Recent Developments
- Challenges in the development of T-cell–based universal influenza vaccines
- Progress and hurdles in the development of influenza virus-like particle vaccines for veterinary use
- New vaccines against influenza virus
- Zoonotic infections with avian influenza A viruses and vaccine preparedness: a game of "mix and match"