J Korean Ophthalmol Soc.
2018 Jan;59(1):9-16. 10.3341/jkos.2018.59.1.9.
Meibography and Immunohistochemistric Structures in Animal Models
- Affiliations
-
- 1Department of Ophthalmology, Korea University College of Medicine, Seoul, Korea. crisim@korea.ac.kr
- 2Department of Ophthalmology, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Korea.
- KMID: 2401672
- DOI: http://doi.org/10.3341/jkos.2018.59.1.9
Abstract
- PURPOSE
We evaluated differences in the meibographic findings and immunohistochemical structures of mice, rats, and rabbits (commonly used in animal experiments).
METHODS
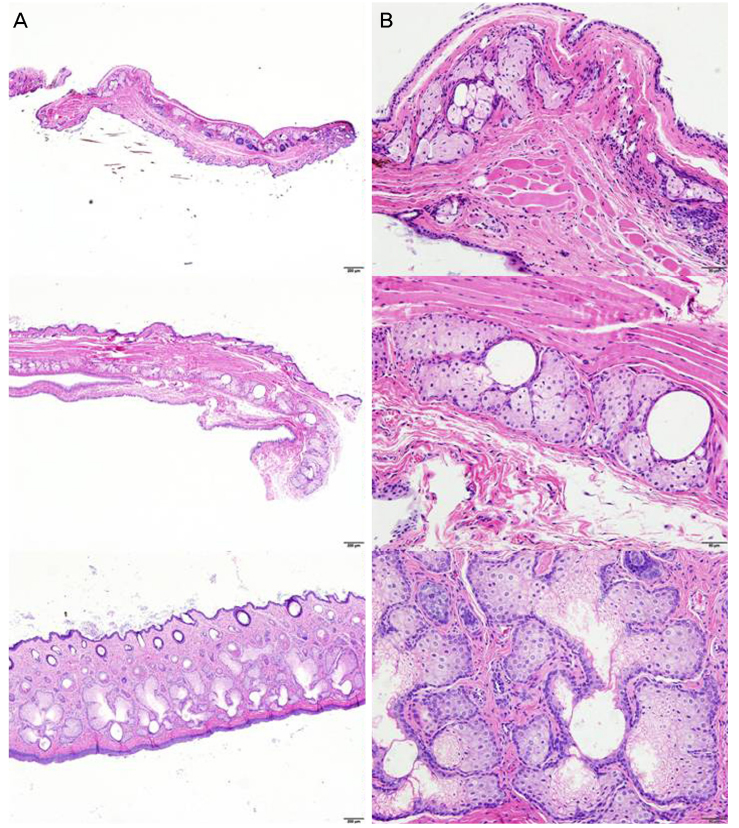
Meibomian gland images were taken using an animal meibograph in mice, rats, and black and white rabbits (five of each animal).The size ratios of the upper and lower Meibomian glands were calculated. Afterm meibographic imaging, the eyes were cut sagittally or transversely and the histological structures were compared using hematoxylin-and-eosin (H&E) and immunohistochemical staining. In addition, the diameters of the central ducts and the areas of ducted glands and the acini were compared using image analysis software.
RESULTS
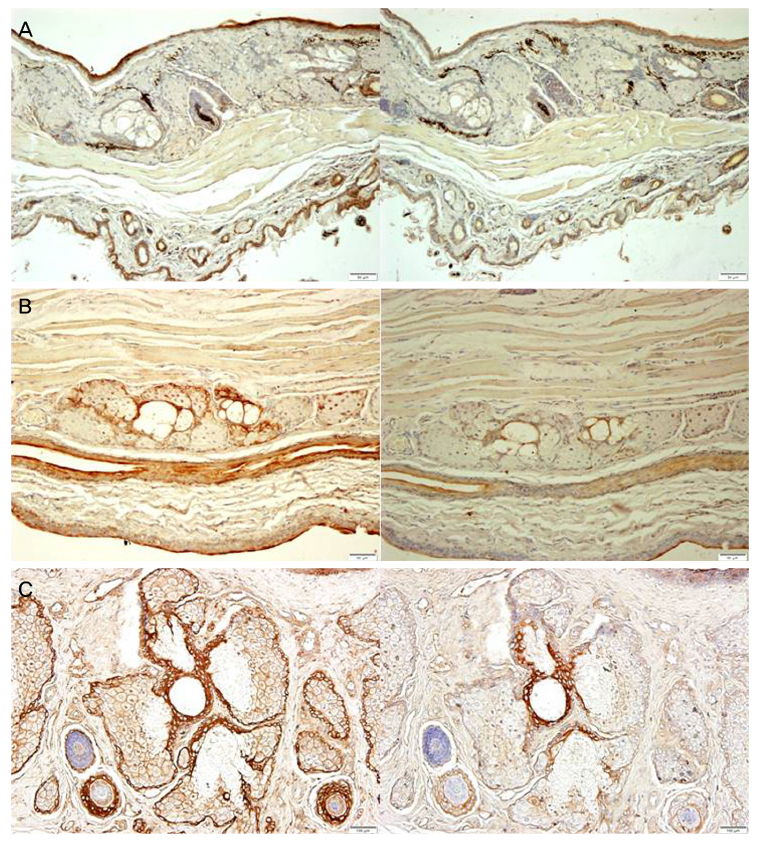
The meibographic findings were clearer in black rabbits than in the other animals. The upper to lower length ratio of the gland did not differ significantly among the three species. Histologically, no central duct epithelial cells were apparent in mice. The diameters of the central ducts and the areas of ducted glands and acini increased in the following order: mouse, rat, and rabbit. However, the Meibomian gland areas did not differ significantly between mice and rats. On immunohistochemical staining, the central ductal epithelium and the Meibomian gland acini were stained for cytokeratin 5 (CK 5) but only the central ductal epithelium was stained for cytokeratin 6 (CK 6).
CONCLUSIONS
The larger the experimental animal, the greater the sizes of the Meibomian gland ducts and acini. In addition, black rabbits yielded better gland images because contrast was enhanced. Compared with sagittal sections of Meibomian glands, transverse sections facilitated a better understanding of the structures of the central ducts and surrounding Meibomian gland acini.
Keyword
MeSH Terms
Figure
Reference
-
1. Jester JV, Rife L, Nii D, et al. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 1982; 22:660–667.2. Baum JL. Biomicroscopic study of the glands of meibomius: by R. Tapie. Ann Ocul 210: 637-648. Surv Ophthalmol. 1979; 23:273–274.3. Killey FP, Minckler DS, Smith RE, Spencer JA. The effect of long term epinephrine in the rabbit eye. In ARVO Abstracts. Invest Ophthalmol Vis Sci. 1980; 19:253.4. Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York: McGraw-Hill Book Co. Inc.;1968. p. 82.5. Jester JV, Nicolaides N, Smith RE. Meibomian gland studies: histologic and ultrastructural investigations. Invest Ophthalmol Vis Sci. 1981; 20:537–547.6. McCulley JP, Sciallis GF. Meibomian keratoconjunctivitis. Am J Ophthalmol. 1977; 84:788–793.
Article7. Nicolaides N, Kaitaranta JK, Rawdah TN, et al. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981; 20:522–536.8. Ehlers N. The precorneal film. Biomicroscopical, histologic, and chemical investigations. Acta Ophthol. 1965; Suppl 81. 1–134.9. Jester JV, Nicolaides N, Kiss-Palvolgyi I, Smith RE. Meibomian gland dysfunction. II. The Role of Kerarinizarion in a rabbit model of MGD. Invest Ophthalmol Vis Sci. 1989; 30:936–945.10. Jester JV, Rajagopalan S, Rodrigues M. Meibomian gland changes in the rhino (hrrhhrrh) mouse. Invest Ophthalmol Vis Sci. 1988; 29:1190–1194.11. Mann SJ. Hair loss and cyst formation in hairless and rhino mutant mice. Anat Rec. 1971; 170:485–499.
Article12. Davies RE, Austin WA, Logani MK. The rhino mutant mouse as an experimental tool. Trans N Y Acad Sci. 1971; 33:680–693.
Article13. Ohnishi Y, Kohno T. Polycholorinated biphenyl poisoning in monkey eye. Invest Ophthalmol Vis Sci. 1979; 18:981–984.14. Gutgesell VJ, Stern GA, Hood CI. Histopathology of meibomian gland dysfunction. Am J Ophthalmol. 1982; 94:383–387.
Article15. Kim JH, Ro JW, Yi K, et al. Changes of the meibomian gland according to age in the normal Korean population. J Korean Ophthalmol Soc. 2015; 56:13–18.
Article16. Butovich IA, Lu H, McMahon A, Eule JC. Toward an animal model of the human tear film: biochemical comparison of the mouse, canine, rabbit, and human meibomian lipidomes. Invest Ophthalmol Vis Sci. 2012; 53:6881–6896.
Article