J Bacteriol Virol.
2017 Dec;47(4):189-198. 10.4167/jbv.2017.47.4.189.
LPS Sensing Mechanism of Human Astrocytes: Evidence of Functional TLR4 Expression and Requirement of Soluble CD14
- Affiliations
-
- 1Department of Microbiology, Institute of Basic Medical Science, Yonsei University, Wonju College of Medicine, Wonju, Gangwon-do, Korea. sun1116@yonsei.ac.kr
- KMID: 2399978
- DOI: http://doi.org/10.4167/jbv.2017.47.4.189
Abstract
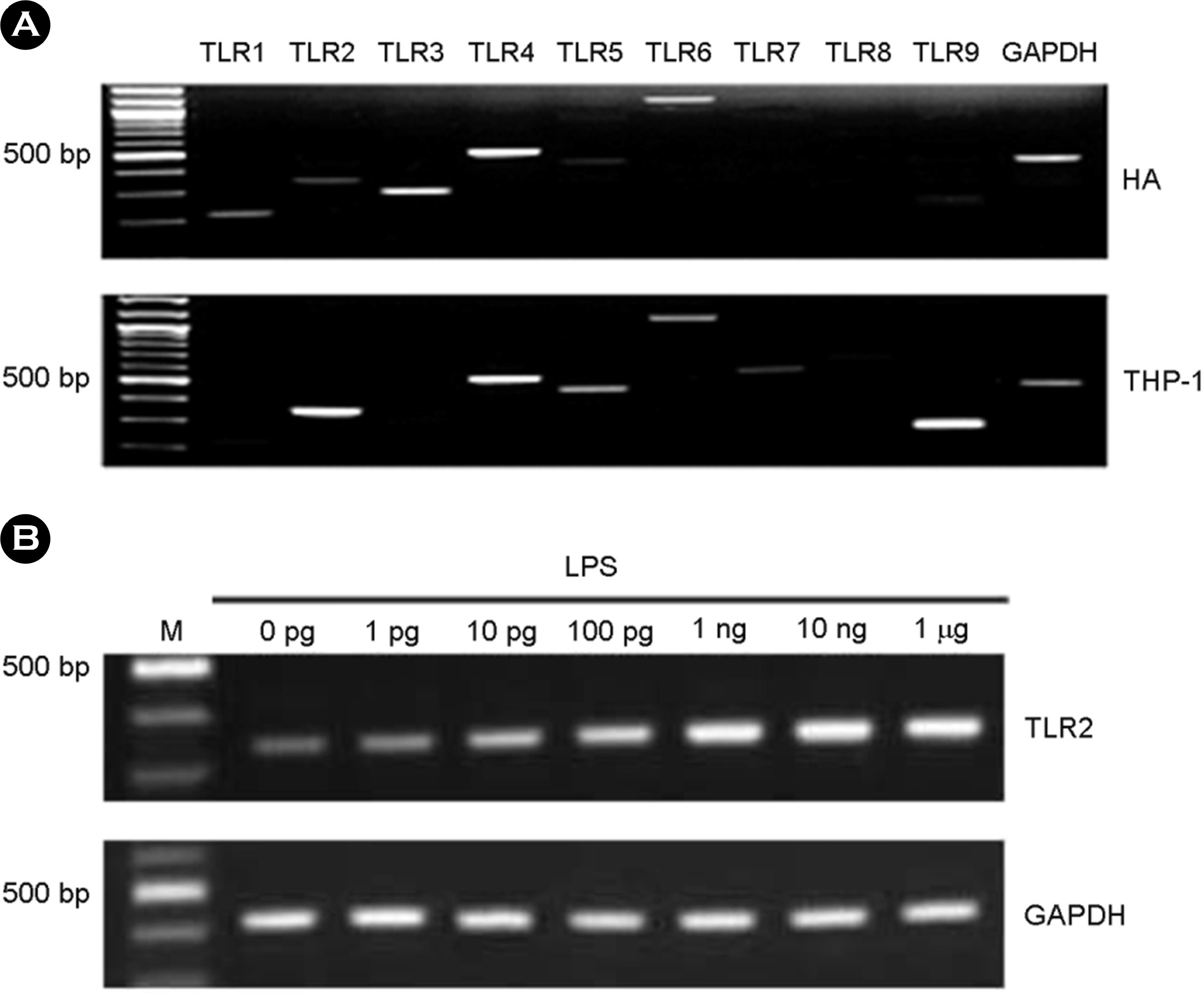
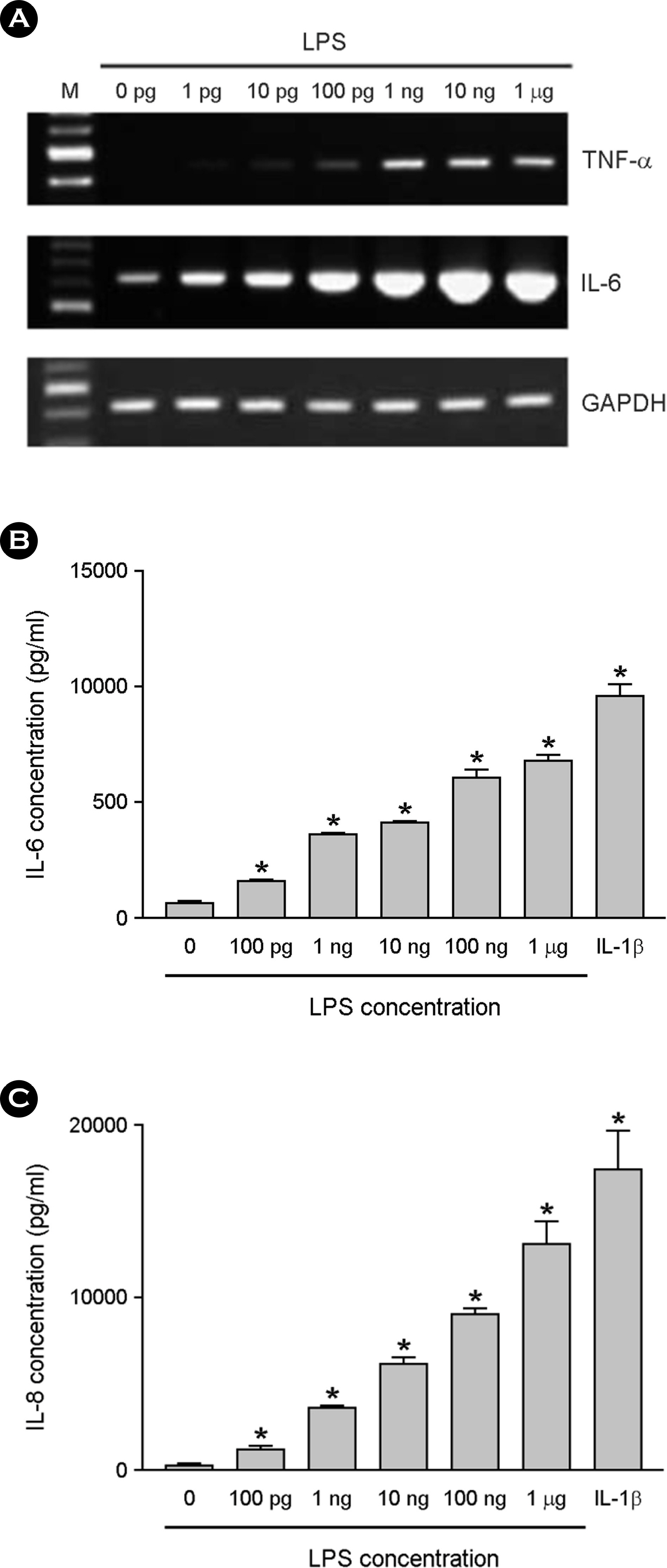
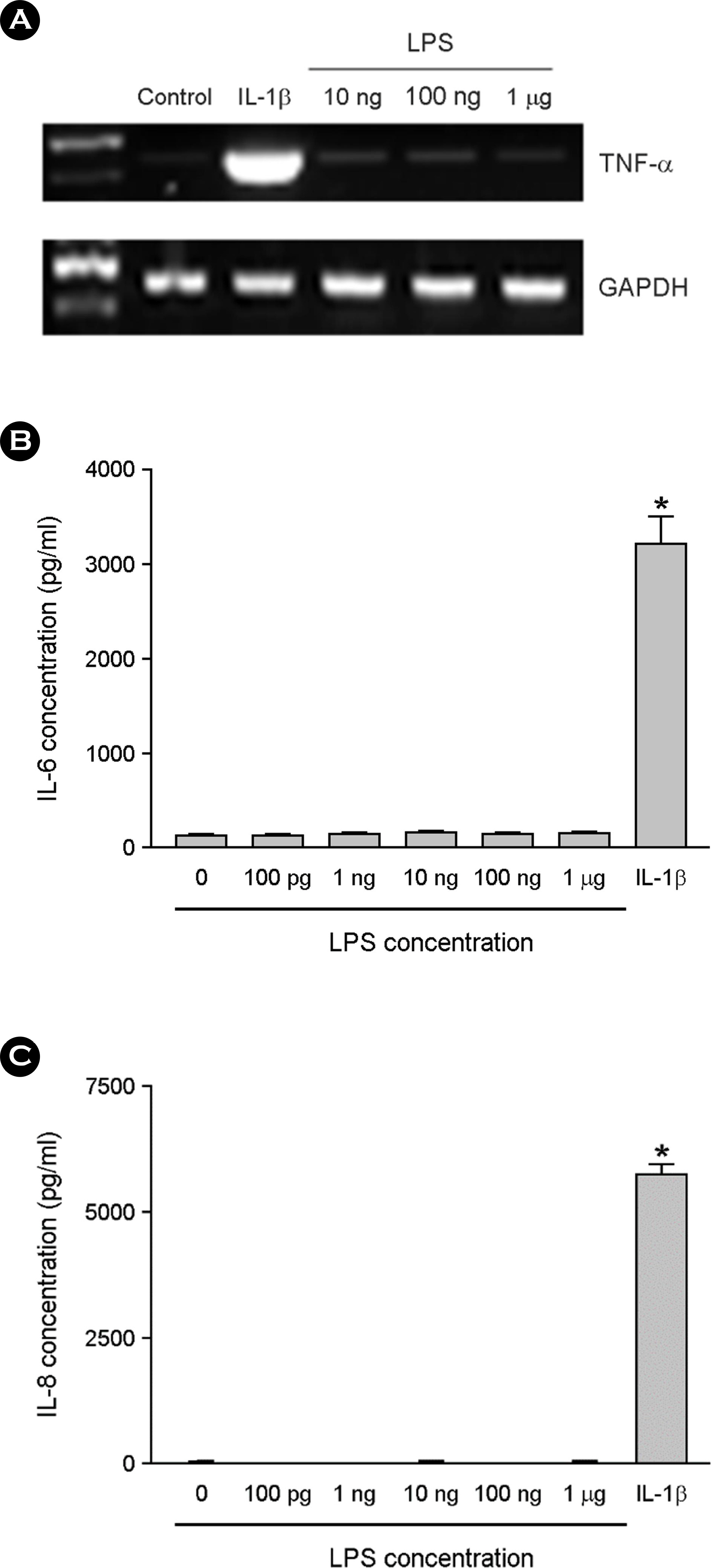
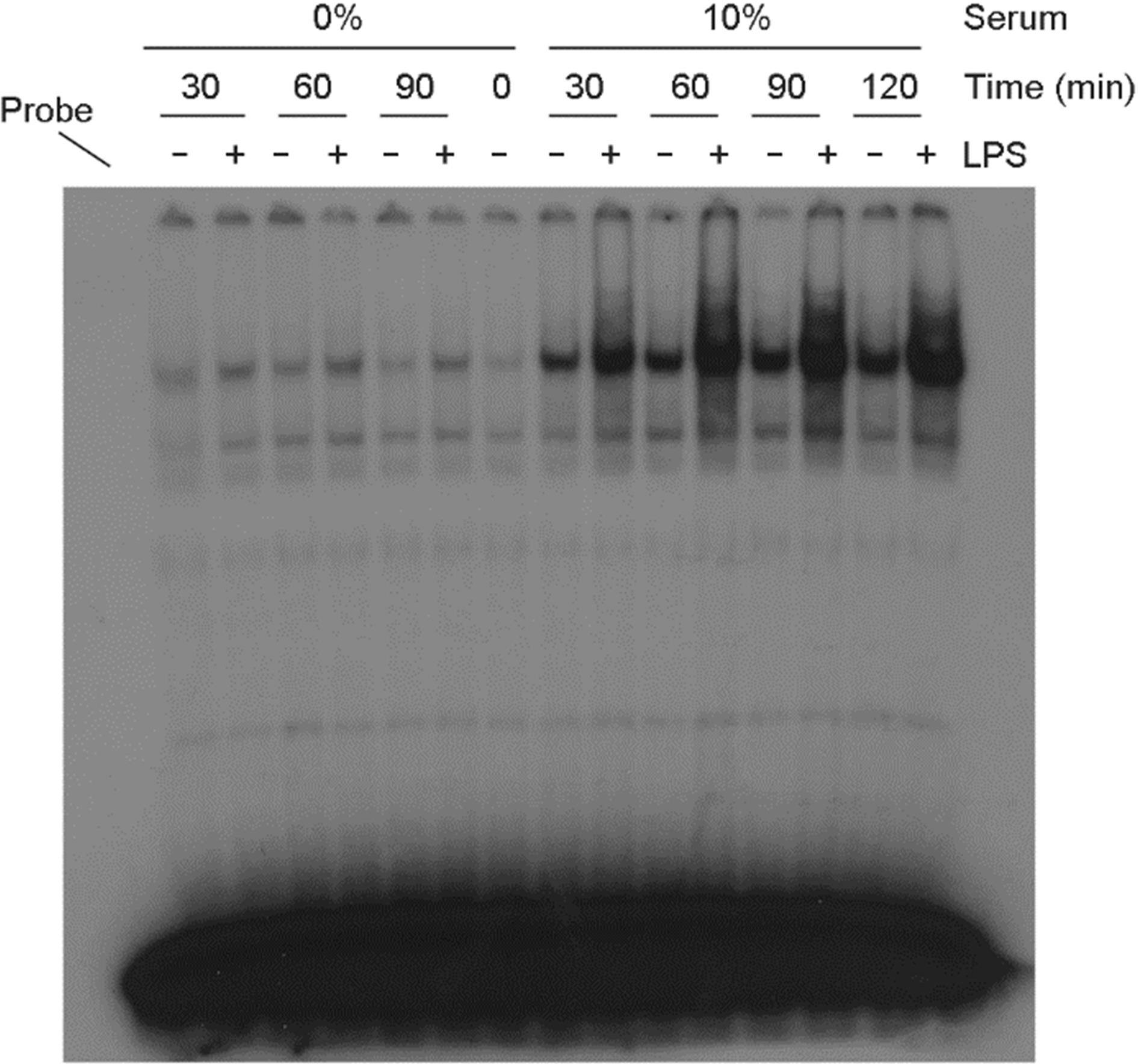
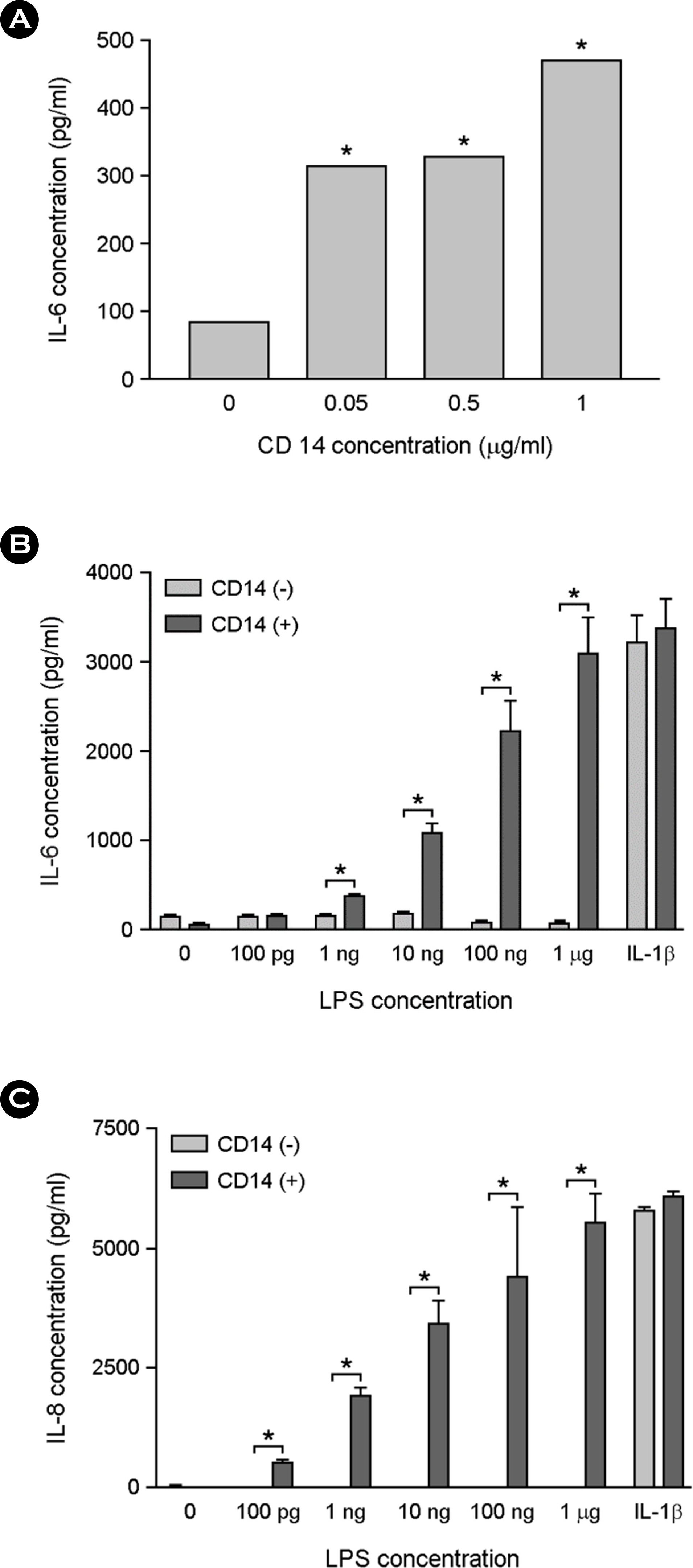
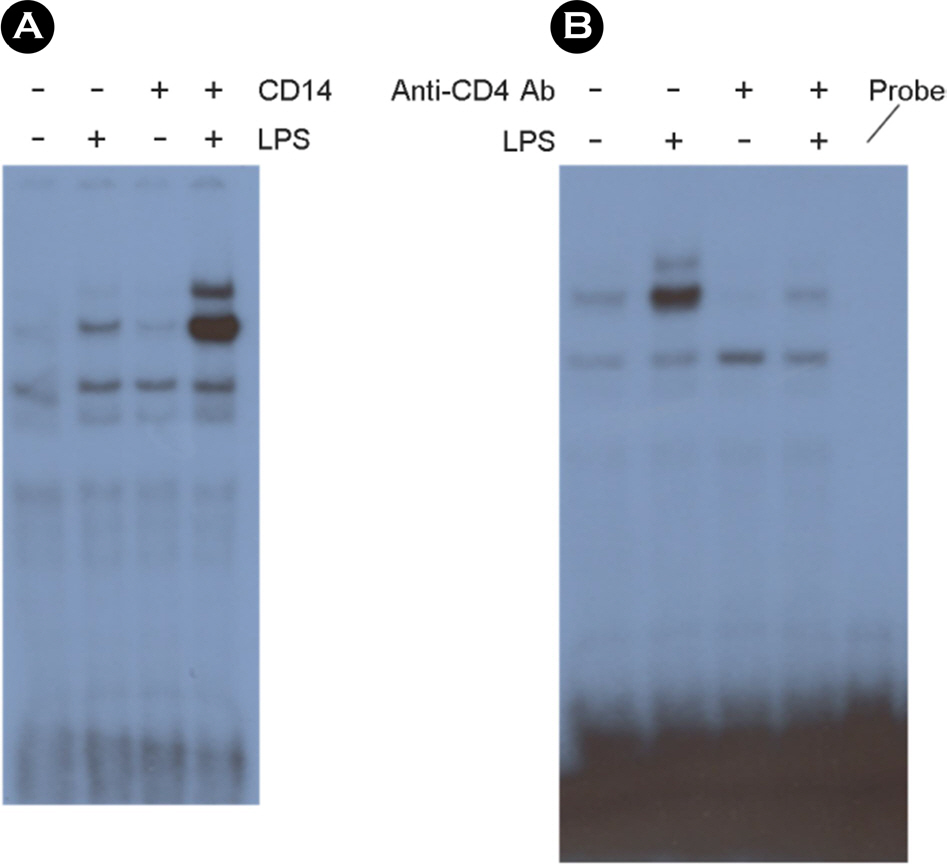
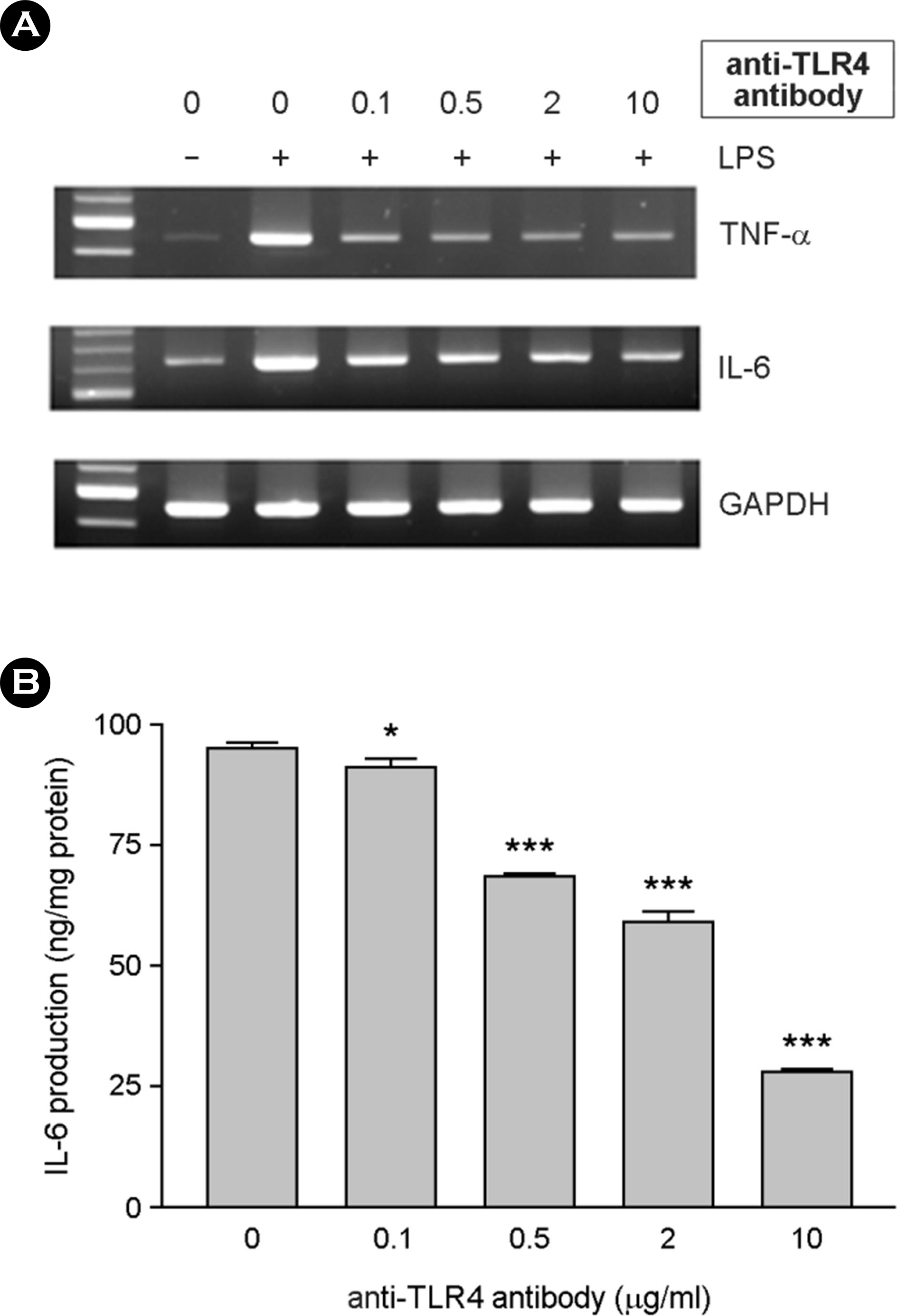
- Among a myriad of pathogen-associated molecular pattern-sensing receptors, toll-like receptors (TLRs) are the principal core sensors of the host. Despite intensive studies for the expression of TLRs and their roles in the central nervous system, controversies remain regarding the expression and the function of TLR4 in human astrocytes. In order to clarify this issue, we attempted to verify functional expression of TLR4 in human astrocytes. Using Reverse transcription-polymerase chain reaction (RT-PCR), we confirmed that the human astrocytes express the TLR4 constitutively. To determine the function of TLR4, astrocytes were treated with TLR4 ligand or lipopolysaccharide (LPS), and then inflammatory cytokines expressions were checked using RT-PCR and enzyme-linked immunosorbent assay. Nuclear factor (NF)-κB activation was checked using electrophoretic mobility shift assay. Treatment of astrocytes with LPS increased tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 expression and induced NF-κB activation. Neutralizing anti-TLR4 antibody blocked the effect of LPS on cytokine production and NF-κB activation in astrocytes. The effect of LPS on cytokine production and NF-κB activation was shown in the presence of serum but not in the absence of serum. Therefore, we investigated the sensing mechanism of LPS in human astrocytes. Human astrocytes were treated with LPS following neutralizing anti-CD14 antibody treatment in the presence of serum. Neutralizing anti-CD14 antibody treatment abolished the effect of LPS on cytokine expression and NF-κB activation. Additionally, supplement of recombinant CD14 in serum-free media induced LPS effect on cytokine production and NF-κB activation. In these results, we showed that human astrocytes constitutively express functional TLR4 and require soluble CD14 to recognize LPS.
Keyword
MeSH Terms
-
Astrocytes*
Central Nervous System
Culture Media, Serum-Free
Cytokines
Electrophoretic Mobility Shift Assay
Enzyme-Linked Immunosorbent Assay
Humans*
Interleukin-8
Interleukins
Toll-Like Receptors
Tumor Necrosis Factor-alpha
Culture Media, Serum-Free
Cytokines
Interleukin-8
Interleukins
Toll-Like Receptors
Tumor Necrosis Factor-alpha
Figure
Reference
-
1). Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Disproportionate recruitment of CD8+ T cells into the central nervous system by professional antigen-presenting cells. Am J Pathol. 1999; 154:481–94.
Article2). Hua LL, Lee SC. Distinct patterns of stimulus-inducible chemokine mRNA accumulation in human fetal astrocytes and microglia. Glia. 2000; 30:74–81.
Article3). Lee SC, Dickson DW, Liu W, Brosnan CF. Induction of nitric oxide synthase activity in human astrocytes by interleukin-1 beta and interferon-gamma. J Neuroimmunol. 1993; 46:19–24.4). Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003; 43:281–91.
Article5). Kielian T. Overview of toll-like receptors in the CNS. Curr Top Microbiol Immunol. 2009; 336:1–14.
Article6). Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005; 49:360–74.
Article7). Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005; 159:12–9.
Article8). Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002; 22:2478–86.
Article9). Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001; 2:675–80.
Article10). Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004; 16:3–9.
Article11). Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005; 17:1–14.12). Bohr V, Paulson OB, Rasmussen N. Pneumococcal meningitis. Late neurologic sequelae and features of prognostic impact. Arch Neurol. 1984; 41:1045–9.13). Dodge PR. Neurological sequelae of acute bacterial meningitis. Pediatr Ann. 1994; 23:101–6.
Article14). Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993; 328:21–8.15). Quagliarello V, Scheld WM. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992; 327:864–72.
Article16). Holm TH, Draeby D, Owens T. Microglia are required for astroglial Toll-like receptor 4 response and for optimal TLR2 and TLR3 response. Glia. 2012; 60:630–8.
Article17). Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003; 100:8514–9.
Article18). Hao HN, Zhao J, Lotoczky G, Grever WE, Lyman WD. Induction of human beta-defensin-2 expression in human astrocytes by lipopolysaccharide and cytokines. J Neurochem. 2001; 77:1027–35.19). Lafortune L, Nalbantoglu J, Antel JP. Expression of tumor necrosis factor alpha (TNF alpha) and interleukin 6 (IL-6) mRNA in adult human astrocytes: comparison with adult microglia and fetal astrocytes. J Neuropathol Exp Neurol. 1996; 55:515–21.20). Lee SS, Seo HS, Choi SJ, Park HS, Lee JY, Lee KH, et al. Characterization of the two genes differentially expressed during development in human fetal astrocytes. Yonsei Med J. 2003; 44:1059–68.
Article21). Choi C, Park JY, Lee J, Lim JH, Shin EC, Ahn YS, et al. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. J Immunol. 1999; 162:1889–95.22). Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983; 11:1475–89.
Article23). Benveniste EN, Benos DJ. TNF-alphaand IFN-gamma-mediated signal transduction pathways: effects on glial cell gene expression and function. FASEB J. 1995; 9:1577–84.24). Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992; 263:C1–16.
Article25). Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002; 61:1013–21.
Article26). Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005; 175:4320–30.
Article27). de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996; 64:37–43.
Article28). Hauss-Wegrzyniak B, Lukovic L, Bigaud M, Stoeckel ME. Brain inflammatory response induced by intracerebroventricular infusion of lipopolysaccharide: an immunohistochemical study. Brain Res. 1998; 794:211–24.
Article29). Mayhan WG. Cellular mechanisms by which tumor necrosis factor-alpha produces disruption of the blood-brain barrier. Brain Res. 2002; 927:144–52.30). Schumann RR, Pfeil D, Freyer D, Buerger W, Lamping N, Kirschning CJ, et al. Lipopolysaccharide and pneumococcal cell wall components activate the mitogen activated protein kinases (MAPK) erk-1, erk-2, and p38 in astrocytes. Glia. 1998; 22:295–305.
Article31). Cauwels A, Frei K, Sansano S, Fearns C, Ulevitch R, Zimmerli W, et al. The origin and function of soluble CD14 in experimental bacterial meningitis. J Immunol. 1999; 162:4762–72.32). Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006; 53:688–95.
Article33). Pugin J, Heumann ID, Tomasz A, Kravchenko VV, Akamatsu Y, Nishijima M, et al. CD14 is a pattern recognition receptor. Immunity. 1994; 1:509–16.
Article34). Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996; 64:1762–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Toll-like Receptor 4 on Human Keratinocytes by Lipoteichoic Acid
- Expression of Microbial Receptors and Production of Cytokine in Mouse Keratinocyte Cells in Response to Lipopolysaccharide Stimulation
- Effect of CD14, Toll-like receptors, cytoskeletal inhibitors and NF-kappaB inhibitor on MMP-8 release from human neutrophils induced by E. coli lipopolysaccharides
- Recognition of lipopolysaccharide pattern by TLR4 complexes
- Expression of Toll-like Receptors on the Macrophages Activated by Bacterial Superantigens