J Rhinol.
2017 Nov;24(2):104-111. 10.18787/jr.2017.24.2.104.
Comparison of Manuka, Kanuka, and Black Locust Honey on the Production of Chemical Mediators by Peripheral Blood Mononuclear Cells
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, School of Medicine, Catholic University of Daegu, Daegu, Korea. hsseung@cu.ac.kr
- KMID: 2398828
- DOI: http://doi.org/10.18787/jr.2017.24.2.104
Abstract
- BACKGROUND AND OBJECTIVES
Honey has various biological and pharmacological activities and has been used as treatment against various inflammatory diseases. The aim of this study was to compare the anti-inflammatory characteristics of manuka, kanuka, and black locust honey. MATERIALS AND METHOD: Peripheral blood mononuclear cells (PBMCs) from healthy human volunteers were isolated and then stimulated with lipopolysaccharide (LPS) with or without pre-treatment of various concentrations of honey for 72 hours. The cytotoxic effects of honeys were measured using an aqueous cell proliferation kit, and the supernatants were analyzed for interleukin-5 (IL-5), IL-10, interferon-γ (INF-γ), and tumor necrosis factor-α (TNF-α) using an enzyme-linked immunosorbent assay.
RESULTS
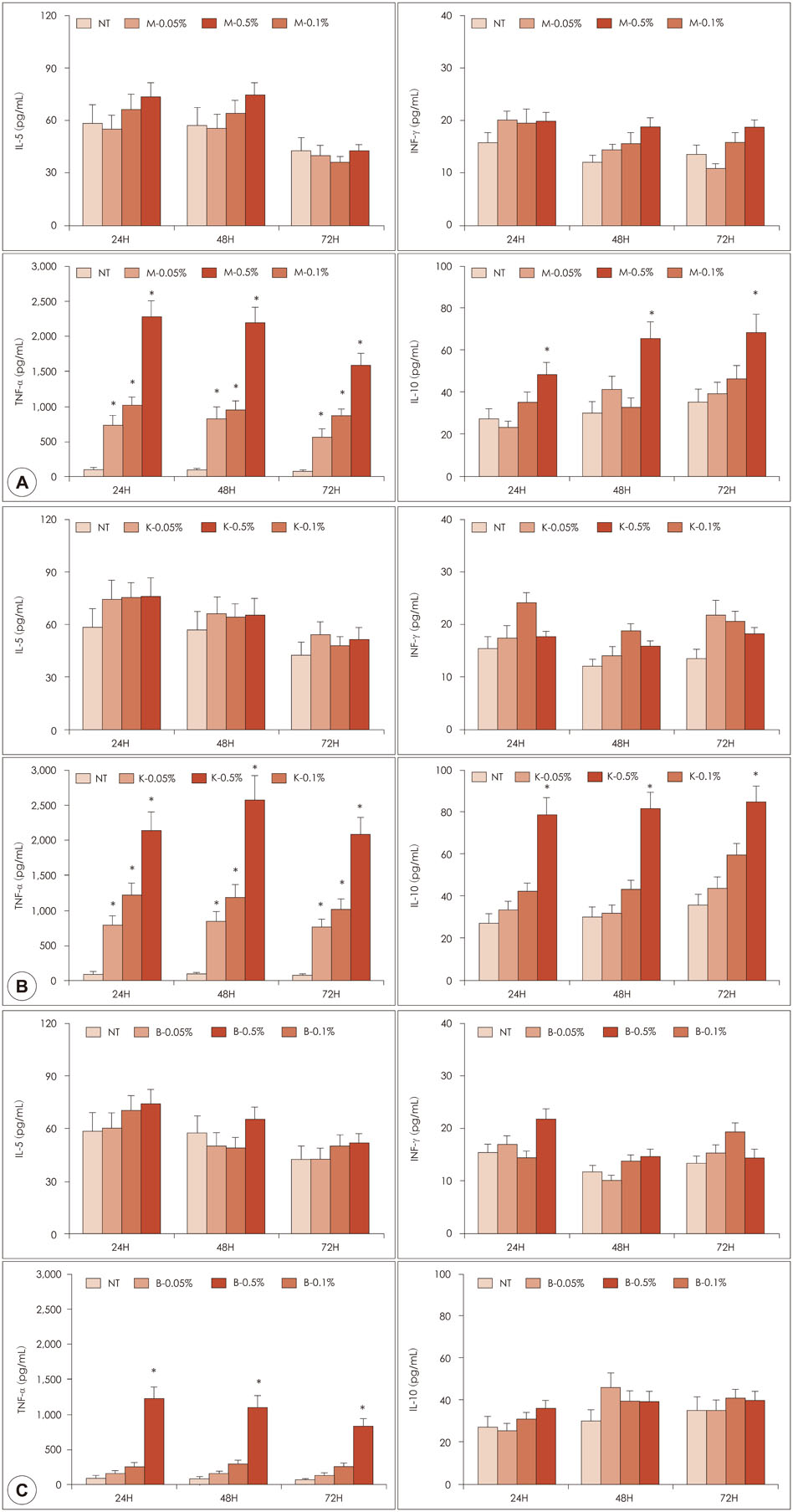
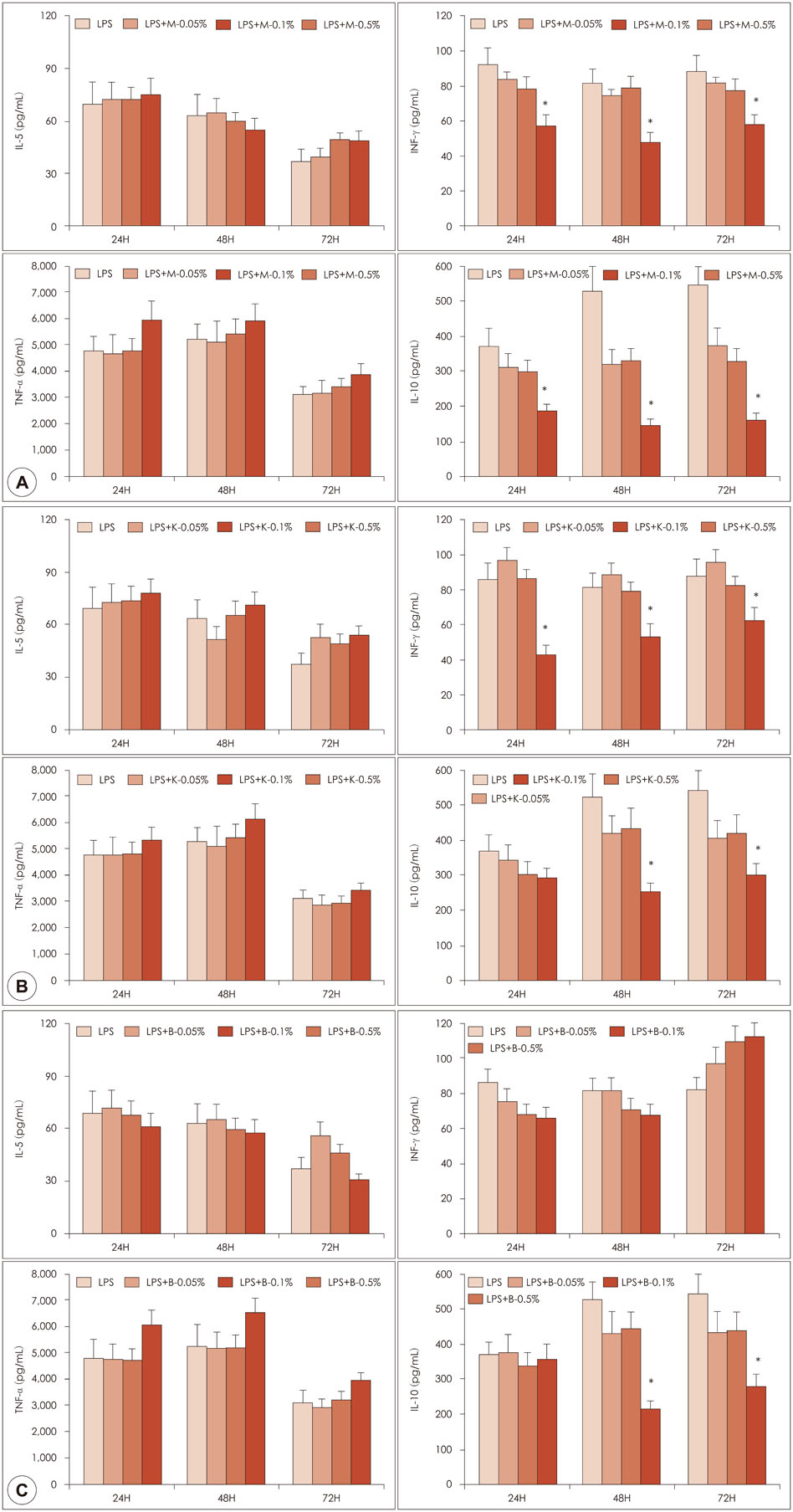
Samples of 1% manuka and kanuka honey were found to have cytotoxic effects on PBMCs. Honey itself enhanced the production of IL-10 and TNF-α production. Manuka and kanuka honeys suppressed LPS-induced IL-10 and INF-γ production, while black locust honey only suppressed IL-10 production from PBMCs.
CONCLUSION
Honeys had immunomodulatory properties of both immunostimulatory and immunosuppressive effects on PBMCs. Different honeys might have different immune modulatory functions due to their different components.
Keyword
MeSH Terms
Figure
Reference
-
1. Zumla A, Lulat A. Honey - a remedy rediscovered. J R Soc Med. 1989; 82:384–385.
Article2. Alandejani T, Marsan J, Ferris W, Slinger R, Chan F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol Head Neck Surg. 2009; 141:114–118.
Article3. Leong AG, Herst PM, Harper JL. Indigenous New Zealand honeys exhibit multiple anti-inflammatory activities. Innate Immun. 2012; 18:459–466.
Article4. Gannabathula S, Skinner MA, Rosendale D, Greenwood JM, Mutukumira AN, Stinhorn G, et al. Arabinogalactan proteins contributes to the immunostimulatory properties of New Zealand honeys. Immunopharmacol Immunotoxicol. 2012; 34:598–607.
Article5. Weston RJ, Mitchell KR, Allen KL. Antibacterial phenolic components of New Zealand manuka honey. Food Chem. 1999; 64:295–301.
Article6. Prakash A, Medhi B, Avti PK, Saikia UN, Pandhi P, Khanduja KL. Effect of different doses of Manuka honey in experimentally induced inflammatory bowel disease in rats. Phytother Res. 2008; 22:1511–1519.
Article7. Nasuti C, Gabbianelli R, Falcioni G, Cantalamessa F. Antioxidative and gastroprotective activities of anti-inflammatory formulations derived from chestnut honey in rats. Nutr Res. 2006; 26:130–137.
Article8. Biswal BM, Zakaria A, Ahmad NM. Topical application of honey in the management of radiation mucositis: a preliminary study. Support Care Cancer. 2003; 11:242–248.
Article9. Cha YH, Bang KS. Studies on the antibacterial activity of honey I. antibacterial activity of balack locust honey. Korean J Apiculture. 1999; 14:85–96.10. Mesaik MA, Azim MK, Mohiuddin S. Honey modulates oxidative burst of professional phagocytes. Phytother Res. 2008; 22:1404–1408.
Article11. Migdal W, Owczarczyk HB, Kedzia B, Holderna-Kedzia E, Madajczyk D. Microbiological decontamination of natural honey by irradiation. Radiat Phys Chem. 2007; 57:285–288.
Article12. Molan PC, Allen KL. The effect of gamma-irradiation on the antibacterial activity of honey. J Pharm Pharmacol. 1996; 48:1206–1209.
Article13. Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002; 3:1129–1134.
Article14. Tonks AJ, Dudley E, Porter NG, Parton J, Brazier J, Smith EL, et al. A 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J Leukoc Biol. 2007; 82:1147–1155.
Article15. Erejuwa OO, Sulaiman SA, Wahab MS. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules. 2014; 19:2497–2522.
Article16. van den Berg AJ, van den Worm E, van Ufford HCQ, Halkes SBA, Hoekstra MJ, Beukelman CJ. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008; 17:172–178. 176–178.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Manuka Honey on Transforming Growth Factor Beta-1-Induced Extracelluar Matrix Production in Nasal Polyp Derived Fibroblasts
- Functional Changes of Interleukin-4 and Interferon-gamma of Peripheral Blood and Nasal Mucosa after Immunotherapy in Patients with Perennial Allergic Rhinitis
- Anti-Inflammatory Effects of Chamaecyparis Obtusa Essential Oil on Peripheral Blood Mononuclear Cells
- Interaction between Peripheral Blood Immune Cells and Activated Respiratory Epithelial Cells with Airborne Fungi
- Manuka Honey versus Antibiotic Ear Drops in Healing of Post-Operative Mastoid Cavity: A Prospective Randomized Trial