Asia Pac Allergy.
2017 Jan;7(1):19-28. 10.5415/apallergy.2017.7.1.19.
The transition of sputum inflammatory cell profiles is variable in stable asthma patients
- Affiliations
-
- 1Division of Allergy and Clinical Immunology, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. yscho@amc.seoul.kr
- KMID: 2396910
- DOI: http://doi.org/10.5415/apallergy.2017.7.1.19
Abstract
- BACKGROUND
The sputum inflammatory cell profile is an important indicator for classifying asthma phenotypes.
OBJECTIVE
To investigate if sputum inflammatory cell profile remains stable and there are different characteristics between groups that show different profile over time in stable asthmatic patients.
METHODS
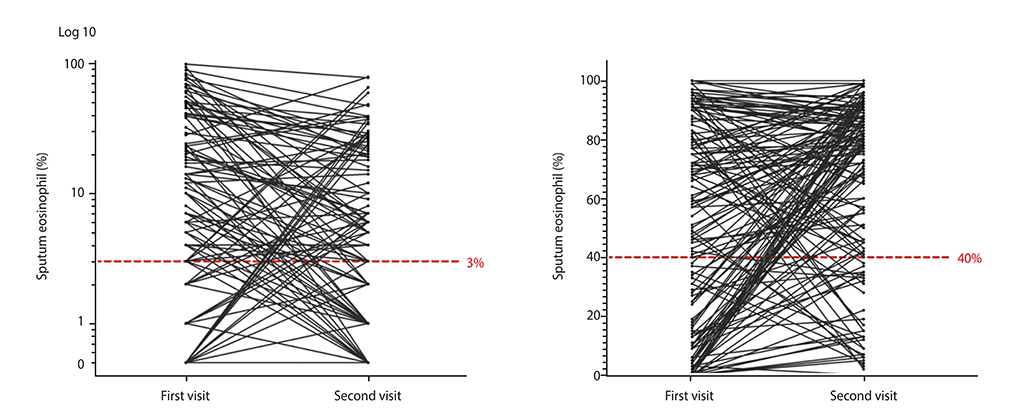
A total of 149 asthmatic patients, who were clinically stable at the time of sputum examination and had undergone sputum analysis twice, were subjected to a detailed review. Eosinophilic inflammation was diagnosed when the proportion of the sputum eosinophils was >3%. We divided the patients into 4 groups according to the transition patterns of their sputum profiles: group 1, persistent eosinophilia; group 2, eosinophilic to noneosinophilic; group 3, noneosinophilic to eosinophilic; and group 4, persistent noneosinophilia. The results of the pulmonary function tests and other clinical parameters were compared between these 4 groups.
RESULTS
Thirty-four of the initially eosinophilic asthmatic patients (39.5%; 34 of 86 patients) demonstrated noneosinophilic airway inflammation at their second sputum examination, and 24 of the initially noneosinophilic patients (38.1%; 24 of 63 patients) demonstrated eosinophilic airway inflammation at follow-up. Various clinical parameters, except the blood eosinophil count, demonstrated no significant differences between the eosinophilic and noneosinophilic asthmatic patients or among the 4 groups.
CONCLUSION
A substantial proportion of asthmatic patients who demonstrate a certain sputum inflammatory cell profile at the initial examination demonstrated profile transition in clinically stable settings over time. The clinical significance of using induced sputum analysis to phenotype stable asthmatic patients requires further evaluation.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Asia Pacific allergy: 6 years old
Yoon-Seok Chang
Asia Pac Allergy. 2017;7(1):1-2. doi: 10.5415/apallergy.2017.7.1.1.
Reference
-
1. Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999; 160:1001–1008.
Article2. Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006; 11:54–61.3. Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999; 353:2213–2214.4. ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. “Refractory” eosinophilic airway inflammation in severe asthma: effect of parenteral corticosteroids. Am J Respir Crit Care Med. 2004; 170:601–605.5. Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007; 370:1422–1431.
Article6. Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, Robinson D, Wenzel S, Busse W, Hansel TT, Barnes NC. International Mepolizumab Study Group. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007; 176:1062–1071.
Article7. Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000; 356:2144–2148.8. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, Chanez P. MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014; 371:1198–1207.
Article9. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 380:651–659.
Article10. Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, Mosesova S, Eisner MD, Bohen SP, Matthews JG. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–1098.
Article11. Gauvreau GM, Boulet LP, Cockcroft DW, Fitzgerald JM, Carlsten C, Davis BE, Deschesnes F, Duong M, Durn BL, Howie KJ, Hui L, Kasaian MT, Killian KJ, Strinich TX, Watson RM, Y N, Zhou S, Raible D, O'Byrne PM. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med. 2011; 183:1007–1014.
Article12. Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, She D, Kell C, May RD, Geba GP, Molfino NA. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J. 2013; 41:330–338.
Article13. Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, Wang L, Kirkesseli S, Rocklin R, Bock B, Hamilton J, Ming JE, Radin A, Stahl N, Yancopoulos GD, Graham N, Pirozzi G. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013; 368:2455–2466.
Article14. Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995; 154:4719–4725.15. Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax. 2010; 65:384–390.
Article16. Al-Samri MT, Benedetti A, Préfontaine D, Olivenstein R, Lemière C, Nair P, Martin JG, Hamid Q. Variability of sputum inflammatory cells in asthmatic patients receiving corticosteroid therapy: a prospective study using multiple samples. J Allergy Clin Immunol. 2010; 125:1161–1163.e4.
Article17. Kupczyk M, Dahlén B, Sterk PJ, Nizankowska-Mogilnicka E, Papi A, Bel EH, Chanez P, Howarth PH, Holgate ST, Brusselle G, Siafakas NM, Gjomarkaj M, Dahlén SE. BIOAIR investigators. Stability of phenotypes defined by physiological variables and biomarkers in adults with asthma. Allergy. 2014; 69:1198–1204.
Article18. Majewski S, Ciebiada M, Domagala M, Kurmanowska Z, Gorski P. Short-term reproducibility of the inflammatory phenotype in different subgroups of adult asthma cohort. Mediators Inflamm. 2015; 2015:419039.
Article19. McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, Fahy JV. Asthma Clinical Research Network of the National Heart, Lung, and Blood Institute. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012; 185:612–619.
Article20. van Veen IH, Ten Brinke A, Gauw SA, Sterk PJ, Rabe KF, Bel EH. Consistency of sputum eosinophilia in difficult-to-treat asthma: a 5-year follow-up study. J Allergy Clin Immunol. 2009; 124:615–617. 617.e1–617.e2.
Article21. Pin I, Gibson PG, Kolendowicz R, Girgis-Gabardo A, Denburg JA, Hargreave FE, Dolovich J. Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax. 1992; 47:25–29.
Article22. Gibson PG, Wlodarczyk JW, Hensley MJ, Gleeson M, Henry RL, Cripps AW, Clancy RL. Epidemiological association of airway inflammation with asthma symptoms and airway hyperresponsiveness in childhood. Am J Respir Crit Care Med. 1998; 158:36–41.
Article23. Fahy JV, Boushey HA, Lazarus SC, Mauger EA, Cherniack RM, Chinchilli VM, Craig TJ, Drazen JM, Ford JG, Fish JE, Israel E, Kraft M, Lemanske RF, Martin RJ, McLean D, Peters SP, Sorkness C, Szefler SJ. NHLBI Asthma Clinical Research Network. Safety and reproducibility of sputum induction in asthmatic subjects in a multicenter study. Am J Respir Crit Care Med. 2001; 163:1470–1475.
Article24. Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER. National Heart, Lung, and Blood Institute Severe Asthma Research Program. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010; 125:1028–1036.e13.
Article25. Deykin A, Lazarus SC, Fahy JV, Wechsler ME, Boushey HA, Chinchilli VM, Craig TJ, Dimango E, Kraft M, Leone F, Lemanske RF, Martin RJ, Pesola GR, Peters SP, Sorkness CA, Szefler SJ, Israel E. Asthma Clinical Research Network, National Heart, Lung, and Blood Institute/NIH. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol. 2005; 115:720–727.
Article26. Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002; 360:1715–1721.
Article27. Petsky HL, Cates CJ, Lasserson TJ, Li AM, Turner C, Kynaston JA, Chang AB. A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils). Thorax. 2012; 67:199–208.
Article28. Black PN, Blasi F, Jenkins CR, Scicchitano R, Mills GD, Rubinfeld AR, Ruffin RE, Mullins PR, Dangain J, Cooper BC, David DB, Allegra L. Tial of roxithromycin in subjects with asthma and serological evidence of infection with Chlamydia pneumoniae. Am J Respir Crit Care Med. 2001; 164:536–541.29. Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008; 177:148–155.
Article30. Sutherland ER, King TS, Icitovic N, Ameredes BT, Bleecker E, Boushey HA, Calhoun WJ, Castro M, Cherniack RM, Chinchilli VM, Craig TJ, Denlinger L, DiMango EA, Fahy JV, Israel E, Jarjour N, Kraft M, Lazarus SC, Lemanske RF Jr, Peters SP, Ramsdell J, Sorkness CA, Szefler SJ, Walter MJ, Wasserman SI, Wechsler ME, Chu HW, Martin RJ. National Heart, Lung and Blood Institute's Asthma Clinical Research Network. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol. 2010; 126:747–753.31. Schleich FN, Manise M, Sele J, Henket M, Seidel L, Louis R. Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm Med. 2013; 13:11.
Article32. Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990; 323:1033–1039.
Article33. Nadif R, Siroux V, Oryszczyn MP, Ravault C, Pison C, Pin I, Kauffmann F. Epidemiological study on the Genetics and Environment of Asthma (EGEA). Heterogeneity of asthma according to blood inflammatory patterns. Thorax. 2009; 64:374–380.
Article34. Fowler SJ, Tavernier G, Niven R. High blood eosinophil counts predict sputum eosinophilia in patients with severe asthma. J Allergy Clin Immunol. 2015; 135:822–824.e2.
Article35. Wagener AH, de Nijs SB, Lutter R, Sousa AR, Weersink EJ, Bel EH, Sterk PJ. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015; 70:115–120.36. Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, Peters SP, Meyers DA, Bleecker ER. National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. 2013; 132:72–80.
Article37. Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008; 38:709–750.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inflammatory cells in the induced sputum of asthmatic patients
- The Association of Eosinophilic Airway Inflammation in Mycoplasma pneumonia and Asthma
- Vascular Endothelial Growth Factor and Matrix Metalloproteinase-9 in Acute Asthma
- Clinical characteristics and inflammatory cells in sputum from asthmatics with acute exacerbation
- Change of Inflammatory Markers in Induced Sputum in Suspected Asthmatics by Asthma Treatment