Korean Circ J.
2017 Nov;47(6):823-832. 10.4070/kcj.2017.0157.
Formation and Transformation of Neointima after Drug-eluting Stent Implantation: Insights from Optical Coherence Tomographic Studies
- Affiliations
-
- 1Sanbon Hospital, Wonkwang University College of Medicine, Gunpo, Korea.
- 2Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea. jangys1212@yuhs.ac
- 3Cardiovascular Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 4Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2396474
- DOI: http://doi.org/10.4070/kcj.2017.0157
Abstract
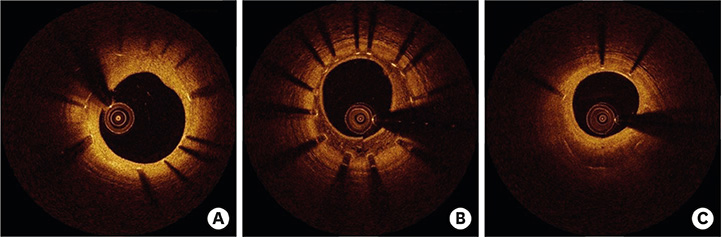
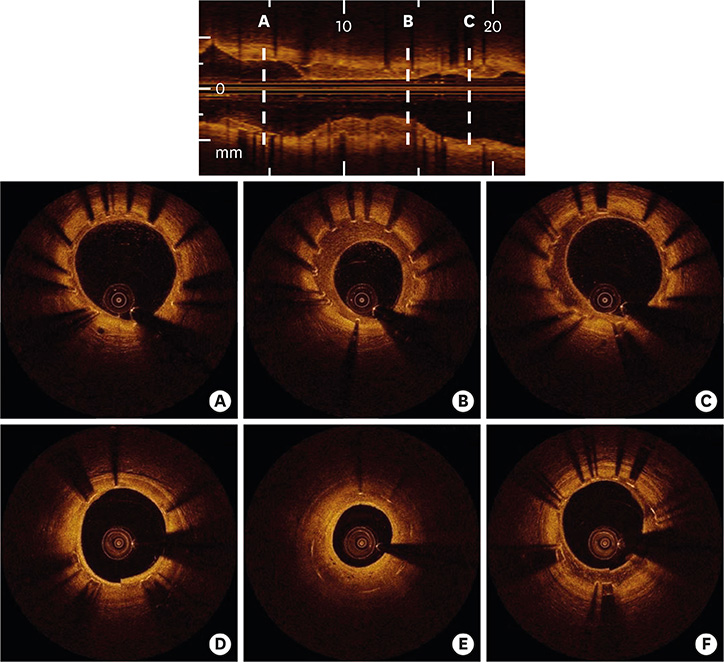
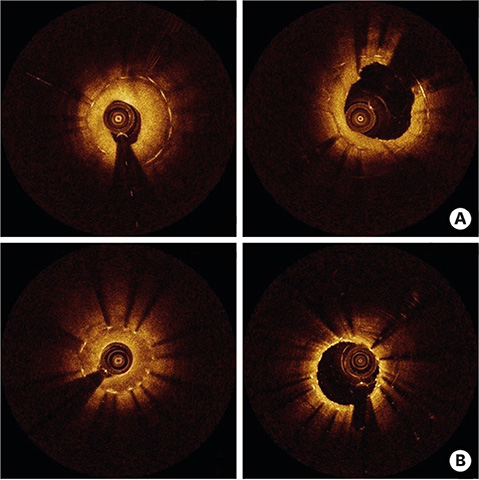
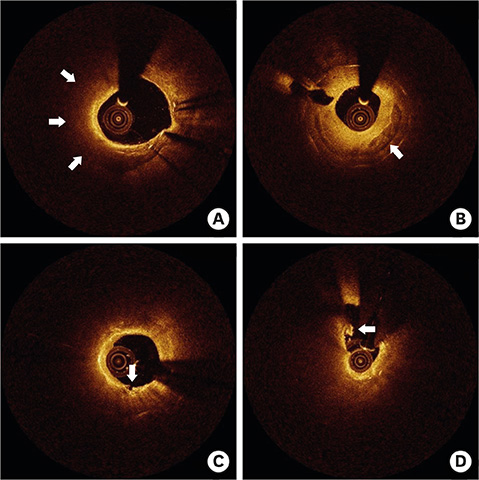
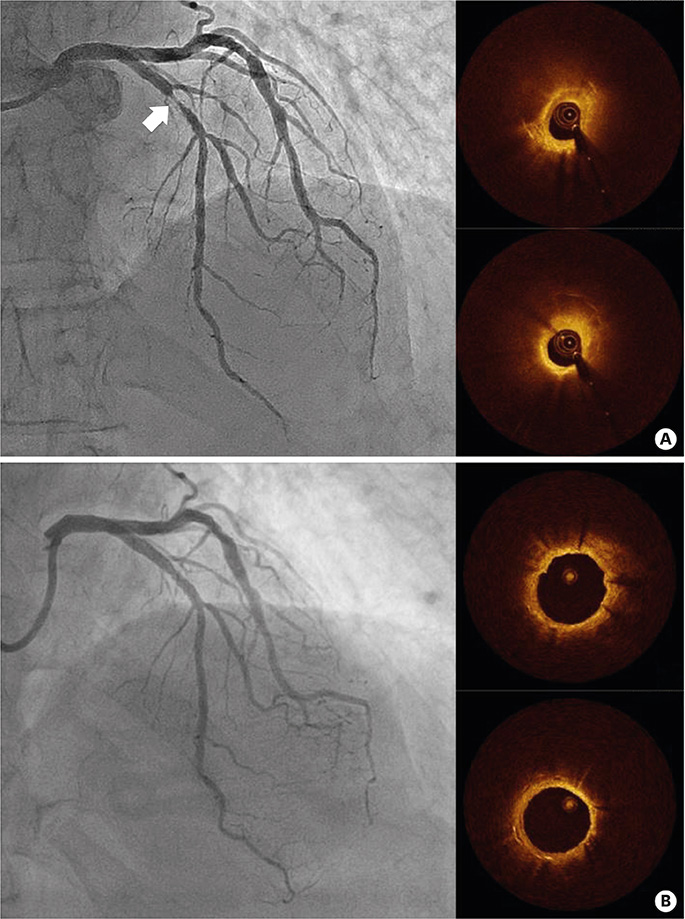
- After coronary stent implantation, neointima formation resembles the wound healing process as it involves the sequential processes of inflammation, granulation, and remodeling. Because antiproliferative drugs and polymers of drug-eluting stents (DESs) delay vascular healing compared with bare metal stents, fibrin deposition can remain long after stent implantation, or inflammation can be excessive. Delayed vascular healing can be associated with adverse clinical outcomes including DES thrombosis or restenosis, and poor endothelization of DES neointima can accelerate neoatherosclerotic change inside the neointima, further contributing to luminal restenosis or neointimal instability. Despite the lack of correlation between pathologic and optical coherence tomography (OCT) findings, OCT assessments of neointima under various circumstances can reveal vascular responses to stent therapy. Homogeneous, heterogeneous, and layered neointima patterns can be recognized by OCT and can change with time. Homogeneous neointima might be associated with better clinical outcomes after DES implantation, whereas non-homogeneous neointima or neoatherosclerotic change can be associated with poorer clinical outcomes. However, limited data are currently available, and further studies are required to comprehensively address these questions.
MeSH Terms
Figure
Cited by 2 articles
-
Stent Selection in Complex Coronary Interventions: Thinking Complex?
Pil Hyung Lee, Seung-Whan Lee
Korean Circ J. 2019;49(1):81-83. doi: 10.4070/kcj.2018.0285.The Effects of Nicotine on Re-endothelialization, Inflammation, and Neoatherosclerosis After Drug-Eluting Stent Implantation in a Porcine Model
Seok Oh, Ju Han Kim, Saleem Ahmad, Yu Jeong Jin, Mi Hyang Na, Munki Kim, Jeong Ha Kim, Dae Sung Park, Dae Young Hyun, Kyung Hoon Cho, Min Chul Kim, Doo Sun Sim, Young Joon Hong, Seung-won Lee, Youngkeun Ahn, Myung Ho Jeong
Korean Circ J. 2024;55(1):50-64. doi: 10.4070/kcj.2024.0171.
Reference
-
1. Kang SJ, Mintz GS, Park DW, et al. Mechanisms of in-stent restenosis after drug-eluting stent implantation: intravascular ultrasound analysis. Circ Cardiovasc Interv. 2011; 4:9–14.2. Cho YK, Hur SH. Practical application of coronary imaging devices in cardiovascular intervention. Korean Circ J. 2015; 45:87–95.3. Farb A, Sangiorgi G, Carter AJ, et al. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999; 99:44–52.4. Nakano M, Virmani R. Histopathology of vascular response to drug-eluting stents: an insight from human autopsy into daily practice. Cardiovasc Interv Ther. 2015; 30:1–11.5. Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006; 48:193–202.6. Otsuka F, Vorpahl M, Nakano M, et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014; 129:211–223.7. Simon C, Palmaz JC, Sprague EA. Influence of topography on endothelialization of stents: clues for new designs. J Long Term Eff Med Implants. 2000; 10:143–151.8. Kolandaivelu K, Swaminathan R, Gibson WJ, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011; 123:1400–1409.9. Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008; 52:333–342.10. Gonzalo N, Serruys PW, Okamura T, et al. Optical coherence tomography patterns of stent restenosis. Am Heart J. 2009; 158:284–293.11. Nakano M, Vorpahl M, Otsuka F, et al. Ex vivo assessment of vascular response to coronary stents by optical frequency domain imaging. JACC Cardiovasc Imaging. 2012; 5:71–82.12. Lutter C, Mori H, Yahagi K, et al. Histopathological differential diagnosis of optical coherence tomographic image interpretation after stenting. JACC Cardiovasc Interv. 2016; 9:2511–2523.13. Lee SY, Shin DH, Kim JS, et al. Optical coherence tomographic observation of morphological features of neointimal tissue after drug-eluting stent implantation. Yonsei Med J. 2014; 55:944–952.14. Lee SY, Hong MK, Mintz GS, et al. Temporal course of neointimal hyperplasia following drug-eluting stent implantation: a serial follow-up optical coherence tomography analysis. Int J Cardiovasc Imaging. 2014; 30:1003–1011.15. Habara M, Terashima M, Nasu K, et al. Morphological differences of tissue characteristics between early, late, and very late restenosis lesions after first generation drug-eluting stent implantation: an optical coherence tomography study. Eur Heart J Cardiovasc Imaging. 2013; 14:276–284.16. Fukuhara K, Okura H, Kume T, Yamada R, Neishi Y, Uemura S. In-stent neointimal characteristics and late neointimal response after drug-eluting stent implantation: a preliminary observation. J Cardiol. 2016; 67:437–441.17. Kimura T, Yokoi H, Nakagawa Y, et al. Three-year follow-up after implantation of metallic coronary-artery stents. N Engl J Med. 1996; 334:561–566.18. Kim JS, Lee JH, Shin DH, et al. Long-term outcomes of neointimal hyperplasia without neoatherosclerosis after drug-eluting stent implantation. JACC Cardiovasc Imaging. 2014; 7:788–795.19. Lee SY, Hong MK, Shin DH, et al. Mechanisms of postintervention and 9-month luminal enlargement after treatment of drug-eluting in-stent restenosis with a drug-eluting balloon. Am J Cardiol. 2014; 113:1468–1473.20. Itoh T, Fusazaki T, Kimura T, et al. Clinical and pathological characteristics of homogeneous and nonhomogeneous tissue of in-stent restenosis visualized by optical coherence tomography. Coron Artery Dis. 2015; 26:201–211.21. Otsuka F, Byrne RA, Yahagi K, et al. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J. 2015; 36:2147–2159.22. Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011; 57:1314–1322.23. Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012; 59:1058–1072.24. Imanaka T, Fujii K, Hao H, et al. Ex vivo assessment of neointimal characteristics after drug-eluting stent implantation: optical coherence tomography and histopathology validation study. Int J Cardiol. 2016; 221:1043–1047.25. Souteyrand G, Amabile N, Mangin L, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J. 2016; 37:1208–1216.26. Taniwaki M, Radu MD, Zaugg S, et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. 2016; 133:650–660.27. Lee SY, Ahn JM, Mintz GS, et al. Characteristics of earlier versus delayed presentation of very late drug-eluting stent thrombosis: an optical coherence tomographic study. J Am Heart Assoc. 2017; 6:e005386.28. Lee SY, Shin DH, Mintz GS, et al. Optical coherence tomography-based evaluation of in-stent neoatherosclerosis in lesions with more than 50% neointimal cross-sectional area stenosis. EuroIntervention. 2013; 9:945–951.29. Kim JS, Hong MK, Shin DH, et al. Quantitative and qualitative changes in DES-related neointimal tissue based on serial OCT. JACC Cardiovasc Imaging. 2012; 5:1147–1155.30. Kimura T, Abe K, Shizuta S, et al. Long-term clinical and angiographic follow-up after coronary stent placement in native coronary arteries. Circulation. 2002; 105:2986–2991.31. Yonetsu T, Kato K, Kim SJ, et al. Predictors for neoatherosclerosis: a retrospective observational study from the optical coherence tomography registry. Circ Cardiovasc Imaging. 2012; 5:660–666.32. Lee SY, Hur SH, Lee SG, et al. Optical coherence tomographic observation of in-stent neoatherosclerosis in lesions with more than 50% neointimal area stenosis after second-generation drug-eluting stent implantation. Circ Cardiovasc Interv. 2015; 8:e001878.33. Otsuka F, Sakakura K, Yahagi K, et al. TCT-655 Contribution of in-stent neoatherosclerosis to late stent failure following bare metal and 1st- and 2nd-generation drug-eluting stent placement: an autopsy study. J Am Coll Cardiol. 2014; 64:B190–B191.34. Mangiameli A, Ohno Y, Attizzani GF, Capodanno D, Tamburino C. Neoatherosclerosis as the cause of late failure of a bioresorbable vascular scaffold. JACC Cardiovasc Interv. 2015; 8:633–634.35. Hiltrop N, Jorge C, Bennett J, Adriaenssens T. Late neoatherosclerotic scaffold failure: an unexpected achilles heel for current bioresorbable scaffold technology? Int J Cardiol. 2016; 223:133–135.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Newly Formed and Ruptured Atheromatous Plaque within Neointima after Drug-Eluting Stent Implantation: 2-Year Follow-Up Intravascular Ultrasound and Optical Coherence Tomography Studies
- Optical Coherence Tomographic Observation of Morphological Features of Neointimal Tissue after Drug-Eluting Stent Implantation
- A Case of Neointimal Calcification in a Drug-Eluting Stent
- Late Stent Thrombosis After Drug-Eluting Stent Implantation: A Rare Case of Accelerated Neo-Atherosclerosis and Early Manifestation of Neointimal Rupture
- Seven Fractures in Three Second Generation Drug Eluting Stents Implanted in the Right Coronary Artery Assessed by Using Optical Coherence Tomography