J Korean Med Sci.
2017 Dec;32(12):1959-1966. 10.3346/jkms.2017.32.12.1959.
Identification of Epstein-Barr Virus in the Human Placenta and Its Pathologic Characteristics
- Affiliations
-
- 1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea. woohokim@snu.ac.kr
- 2Laboratory of Epigenetics, Cancer Research Institute, Seoul National University, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- KMID: 2396372
- DOI: http://doi.org/10.3346/jkms.2017.32.12.1959
Abstract
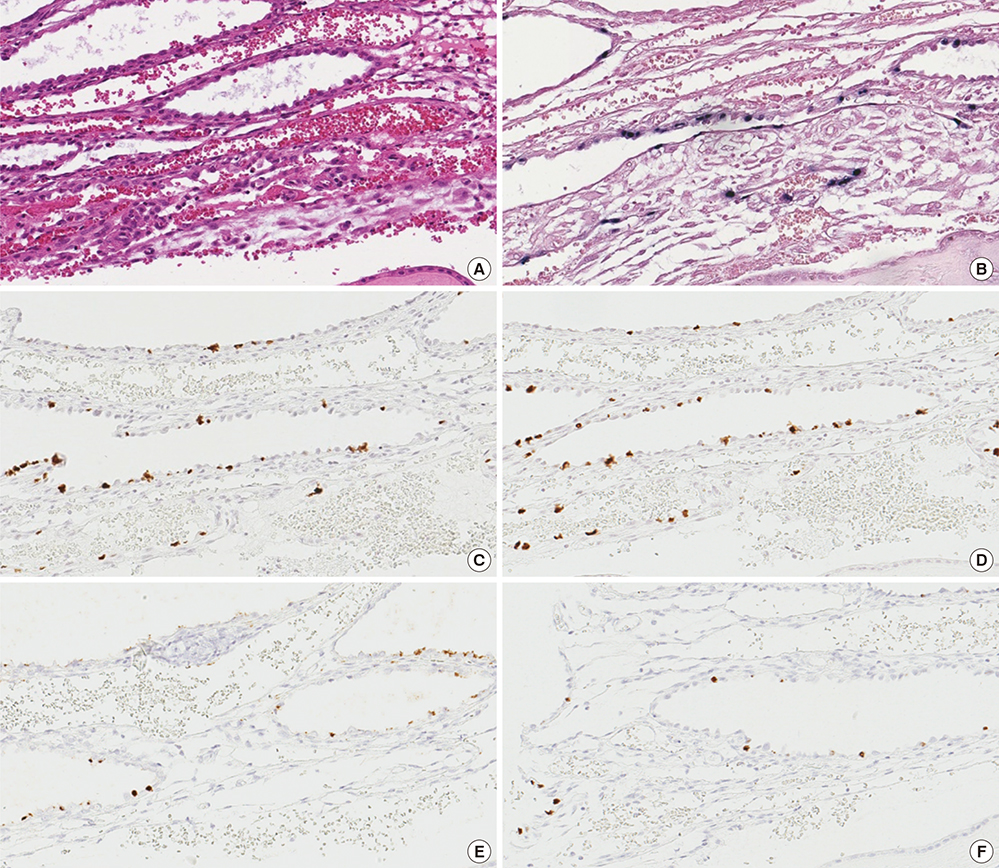
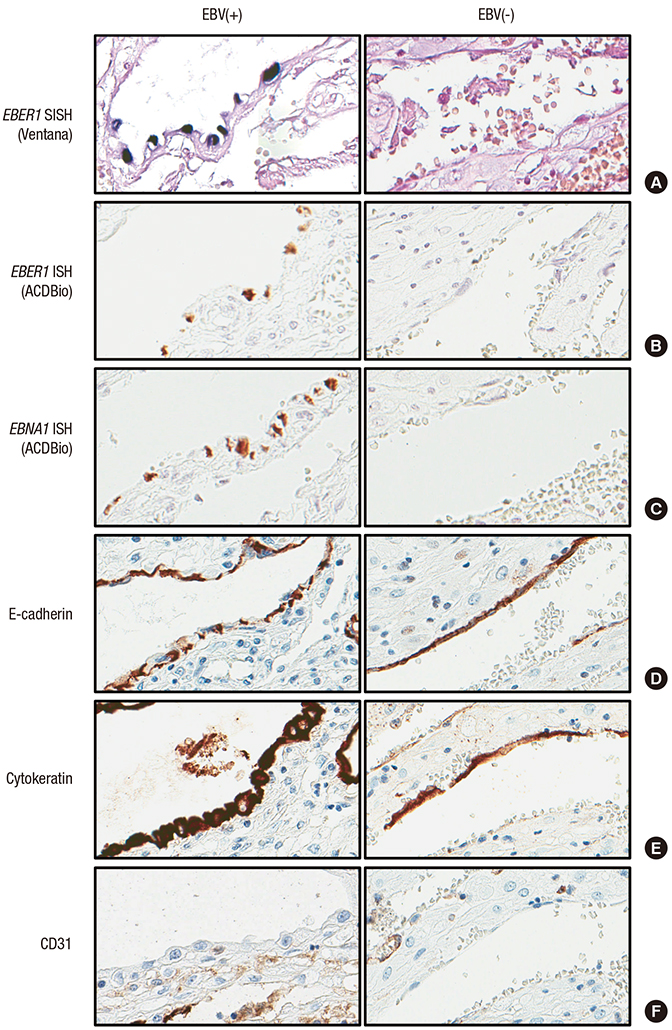
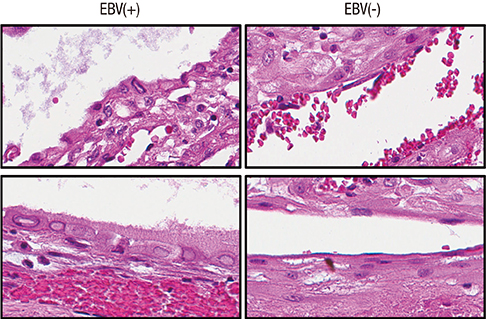
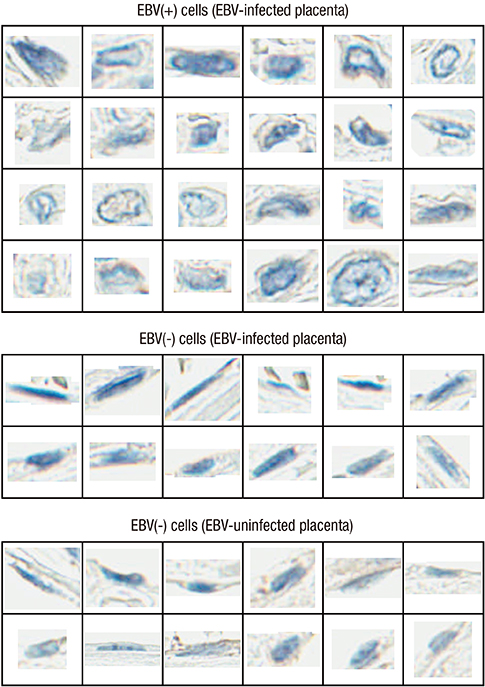
- Epstein-Barr virus (EBV), a common pathogen in humans, is suspected as the cause of multiple pregnancy-related pathologies including depression, preeclampsia, and stillbirth. Moreover, transmission of EBV through the placenta has been reported. However, the focus of EBV infection within the placenta has remained unknown to date. In this study, we proved the expression of latent EBV genes in the endometrial glandular epithelial cells of the placenta and investigated the cytological characteristics of these cells. Sixty-eight placentas were obtained from pregnant women. Tissue microarray was constructed. EBV latent genes including EBV-encoding RNA-1 (EBER1), Epstein-Barr virus nuclear antigen 1 (EBNA1), late membrane antigen (LMP1), and RPMS1 were detected with silver in situ hybridization and/or mRNA in situ hybridization. Nuclear features of EBV-positive cells in EBV-infected placenta were compared with those of EBV-negative cells via image analysis. Sixteen placentas (23.5%) showed positive expression of all 4 EBV latent genes; only the glandular epithelial cells of the decidua showed EBV gene expression. EBV infection status was not significantly correlated with maternal, fetal, or placental factors. The nuclei of EBV-positive cells were significantly larger, longer, and round-shaped than those of EBV-negative cells regardless of EBV-infection status of the placenta. For the first time, evidence of EBV gene expression has been shown in placental tissues. Furthermore, we have characterized its cytological features, allowing screening of EBV infection through microscopic examination.
Keyword
MeSH Terms
Figure
Reference
-
1. Chang MS, Lee HS, Kim CW, Kim YI, Kim WH. Clinicopathologic characteristics of Epstein-Barr virus-incorporated gastric cancers in Korea. Pathol Res Pract. 2001; 197:395–400.2. Fleisher G, Henle W, Henle G, Lennette ET, Biggar RJ. Primary infection with Epstein-Barr virus in infants in the United States: clinical and serologic observations. J Infect Dis. 1979; 139:553–558.3. Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat Med. 2002; 8:594–599.4. Okano M, Gross TG. Acute or chronic life-threatening diseases associated with Epstein-Barr virus infection. Am J Med Sci. 2012; 343:483–489.5. Draborg AH, Duus K, Houen G. Epstein-Barr virus in systemic autoimmune diseases. Clin Dev Immunol. 2013; 2013:535738.6. Tugizov S, Herrera R, Veluppillai P, Greenspan J, Greenspan D, Palefsky JM. Epstein-Barr virus (EBV)-infected monocytes facilitate dissemination of EBV within the oral mucosal epithelium. J Virol. 2007; 81:5484–5496.7. Zhu P, Chen YJ, Hao JH, Ge JF, Huang K, Tao RX, Jiang XM, Tao FB. Maternal depressive symptoms related to Epstein-Barr virus reactivation in late pregnancy. Sci Rep. 2013; 3:3096.8. Elliott SE, Parchim NF, Kellems RE, Xia Y, Soffici AR, Daugherty PS. A pre-eclampsia-associated Epstein-Barr virus antibody cross-reacts with placental GPR50. Clin Immunol. 2016; 168:64–71.9. Tomai XH. Stillbirth following severe symmetric fetal growth restriction due to reactivation of Epstein-Barr virus infection in pregnancy. J Obstet Gynaecol Res. 2011; 37:1877–1882.10. Meyohas MC, Maréchal V, Desire N, Bouillie J, Frottier J, Nicolas JC. Study of mother-to-child Epstein-Barr virus transmission by means of nested PCRs. J Virol. 1996; 70:6816–6819.11. Braz-Silva PH, de Rezende NP, Ortega KL, de Macedo Santos RT, de Magalhães MH. Detection of the Epstein-Barr virus (EBV) by in situ hybridization as definitive diagnosis of hairy leukoplakia. Head Neck Pathol. 2008; 2:19–24.12. Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006; 7:R100.13. Iwasaka T, Kidera Y, Tsugitomi H, Sugimori H. The cellular changes in primary and recurrent infection with herpes simplex virus type 2 in an in vitro model. Acta Cytol. 1987; 31:935–940.14. Juric-Sekhar G, Upton MP, Swanson PE, Westerhoff M. Cytomegalovirus (CMV) in gastrointestinal mucosal biopsies: should a pathologist perform CMV immunohistochemistry if the clinician requests it? Hum Pathol. 2017; 60:11–15.15. Martínez-López JL, Torres J, Camorlinga-Ponce M, Mantilla A, Leal YA, Fuentes-Pananá EM. Evidence of Epstein-Barr virus association with gastric cancer and non-atrophic gastritis. Viruses. 2014; 6:301–318.16. Vincent-Bugnas S, Vitale S, Mouline CC, Khaali W, Charbit Y, Mahler P, Prêcheur I, Hofman P, Maryanski JL, Doglio A. EBV infection is common in gingival epithelial cells of the periodontium and worsens during chronic periodontitis. PLoS One. 2013; 8:e80336.17. Al-Moslih MI, White RJ, Dubes GR. Use of a transfection method to demonstrate a monolayer cell transforming agent from the EB3 line of Burkitt's lymphoma cells. J Gen Virol. 1976; 31:331–345.18. Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005; 202:231–237.19. Prasad S, Hu S, Sheng WS, Chauhan P, Singh A, Lokensgard JR. The PD-1: PD-L1 pathway promotes development of brain-resident memory T cells following acute viral encephalitis. J Neuroinflammation. 2017; 14:82.20. Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006; 27:195–201.21. McNally B, Ye F, Willette M, Flaño E. Local blockade of epithelial PDL-1 in the airways enhances T cell function and viral clearance during influenza virus infection. J Virol. 2013; 87:12916–12924.22. Delorme-Axford E, Sadovsky Y, Coyne CB. The placenta as a barrier to viral infections. Annu Rev Virol. 2014; 1:133–146.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Studies on the Epstein-Barr virus transformed human B-lymphocytes1. flowcytometry analysis of Epstein-Barr virus transformed human B-lymphocytes
- Studies on the Epstein-Barr virus transformed human B-lymphocytes2. production of LT-like factor by Epstein-Barr virus transformed human B-lymphocytes
- A Case of Epstein-Barr Virus-Associated Hemophagocytic Syndrome Demonstrated by In Situ Hybridization
- A Case of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma Occurring in Thyroid Gland
- Several Cases of Epstein-Barr Virus Associated Hepatitis