J Korean Neurosurg Soc.
2017 Nov;60(6):611-619. 10.3340/jkns.2017.0178.
The Formation of Extragraft Bone Bridging after Anterior Cervical Discectomy and Fusion: A Finite Element Analysis
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea. chungc@snu.ac.kr
- 2Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea.
- 3Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 4Department of Brain and Cognitive Sciences, Seoul National University College of Natural Sciences, Seoul, Korea.
- 5Department of Biomedical Engineering, Inje University, Gimhae, Korea.
- 6R&D Center, Medyssey Co., Ltd, Jecheon, Korea.
- KMID: 2395779
- DOI: http://doi.org/10.3340/jkns.2017.0178
Abstract
OBJECTIVE
In addition to bone bridging inside a cage or graft (intragraft bone bridging, InGBB), extragraft bone bridging (ExGBB) is commonly observed after anterior cervical discectomy and fusion (ACDF) with a stand-alone cage. However, solid bony fusion without the formation of ExGBB might be a desirable condition. We hypothesized that an insufficient contact area for InGBB might be a causative factor for ExGBB. The objective was to determine the minimal area of InGBB by finite element analysis.
METHODS
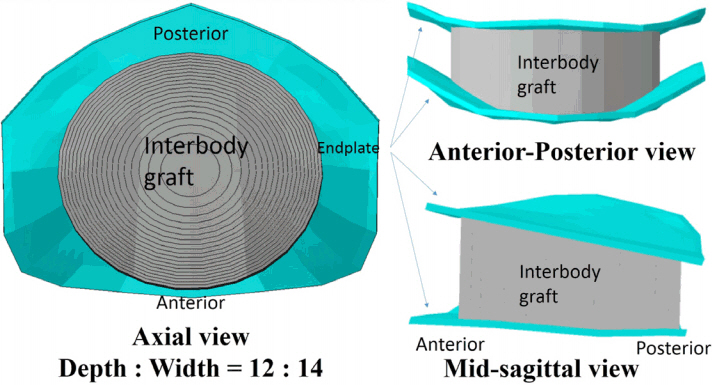
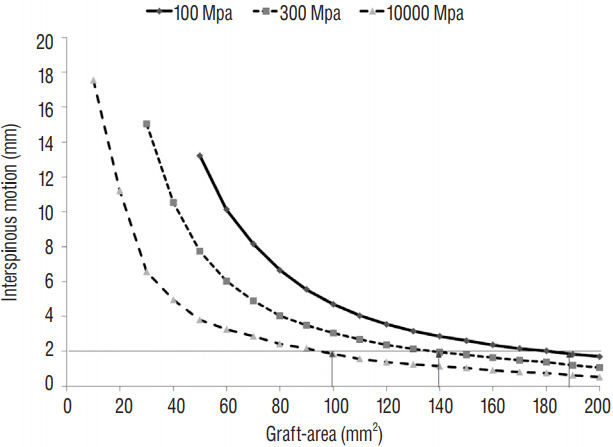
A validated 3-dimensional, nonlinear ligamentous cervical segment (C3-7) finite element model was used. This study simulated a single-level ACDF at C5-6 with a cylindroid interbody graft. The variables were the properties of the incorporated interbody graft (cancellous bone [Young's modulus of 100 or 300 MPa] to cortical bone [10000 MPa]) and the contact area between the vertebra and interbody graft (Graft-area, from 10 to 200 mm²). Interspinous motion between the flexion and extension models of less than 2 mm was considered solid fusion.
RESULTS
The minimal Graft-areas for solid fusion were 190 mm², 140 mm², and 100 mm² with graft properties of 100, 300, and 10000 MPa, respectively. The minimal Graft-areas were generally unobtainable with only the formation of InGBB after the use of a commercial stand-alone cage.
CONCLUSION
ExGBB may be formed to compensate for insufficient InGBB. Although various factors may be involved, solid fusion with less formation of ExGBB may be achieved with refinements in biomaterials, such as the use of osteoinductive cage materials; changes in cage design, such as increasing the area of polyetheretherketone or the inside cage area for bone grafts; or surgical techniques, such as the use of plate/screw systems.
MeSH Terms
Figure
Reference
-
References
1. Buchowski JM, Liu G, Bunmaprasert T, Rose PS, Riew KD. Anterior cervical fusion assessment: surgical exploration versus radiographic evaluation. Spine (Phila Pa 1976). 33:1185–1191. 2008.2. Chong E, Pelletier MH, Mobbs RJ, Walsh WR. The design evolution of interbody cages in anterior cervical discectomy and fusion: a systematic review. BMC Musculoskelet Disord. 16:99. 2015.
Article3. Faizan A, Goel VK, Garfin SR, Bono CM, Serhan H, Biyani A, et al. Do design variations in the artificial disc influence cervical spine biomechanics? A finite element investigation. Eur Spine J. 21(Suppl 5):S653–S662. 2012.
Article4. Floyd T, Ohnmeiss D. A meta-analysis of autograft versus allograft in anterior cervical fusion. Eur Spine J. 9:398–403. 2000.
Article5. Galbusera F, Bellini CM, Costa F, Assietti R, Fornari M. Anterior cervical fusion: a biomechanical comparison of 4 techniques. Laboratory investigation. J Neurosurg Spine. 9:444–449. 2008.6. Galbusera F, Bellini CM, Raimondi MT, Fornari M, Assietti R. Cervical spine biomechanics following implantation of a disc prosthesis. Med Eng Phys. 30:1127–1133. 2008.
Article7. Ha SK. Finite element modeling of multi-level cervical spinal segments (C3–C6) and biomechanical analysis of an elastomer-type prosthetic disc. Med Eng Phys. 28:534–541. 2006.
Article8. Harrison DE, Harrison DD, Cailliet R, Troyanovich SJ, Janik TJ, Holland B. Cobb method or harrison posterior tangent method: which to choose for lateral cervical radiographic analysis. Spine (Phila Pa 1976). 25:2072–2078. 2000.9. Heneghan HM, McCabe JP. Use of autologous bone graft in anterior cervical decompression: morbidity & quality of life analysis. BMC Musculoskelet Disord. 10:158. 2009.
Article10. Jung TG, Woo SH, Park KM, Jang JW, Han DW, Lee SJ. Biomechanical behavior of two different cervical total disc replacement designs in relation of concavity of articular surfaces: ProDisc-C® vs. Prestige-LP® Int J Precis Eng Manuf. 14:819–824. 2013.
Article11. Kaiser MG, Mummaneni PV, Matz PG, Anderson PA, Groff MW, Heary RF, et al. Radiographic assessment of cervical subaxial fusion. J Neurosurg Spine. 11:221–227. 2009.
Article12. Kim CH, Chung CK, Choi Y, Hwang ST, Kim SM, Park SB. The patient-reported outcome of chronic pain after the harvest of anterior iliac bone for anterior cervical arthrodesis. J Clin Neurosci. 36:102–107. 2017.
Article13. Kim CH, Chung CK, Hahn S. Autologous iliac bone graft with anterior plating is advantageous over the stand-alone cage for segmental lordosis in single-level cervical disc disease. Neurosurgery. 72:257–265. discussion 266. 2013.
Article14. Kim CH, Chung CK, Jahng TA, Park SB, Sohn S, Lee S. Segmental kyphosis after cervical interbody fusion with stand-alone polyetheretherketone (PEEK) cages: a comparative study on 2 different PEEK cages. J Spinal Disord Tech. 28:E17–E24. 2015.15. Lee SE, Chung CK, Kim CH. Difference in canal encroachment by the fusion mass between anterior cervical discectomy and fusion with bone autograft and anterior plating, and stand-alone cage. J Clin Neurosci. 29:121–127. 2016.
Article16. Mackiewicz A, Banach M, Denisiewicz A, Bedzinski R. Comparative studies of cervical spine anterior stabilization systems - finite element analysis. Clin Biomech (Bristol, Avon). 32:72–79. 2016.
Article17. Panjabi MM, Crisco JJ, Vasavada A, Oda T, Cholewicki J, Nibu K, et al. Mechanical properties of the human cervical spine as shown by three-dimensional load-displacement curves. Spine (Phila Pa 1976). 26:2692–2700. 2001.
Article18. Pechlivanis I, Thuring T, Brenke C, Seiz M, Thome C, Barth M, et al. Non-fusion rates in anterior cervical discectomy and implantation of empty polyetheretherketone cages. Spine (Phila Pa 1976). 36:15–20. 2011.
Article19. Rhee JM, Chapman JR, Norvell DC, Smith J, Sherry NA, Riew KD. Radiological determination of postoperative cervical fusion: a systematic review. Spine (Phila Pa 1976). 40:974–991. 2015.20. Shriver MF, Lewis DJ, Kshettry VR, Rosenbaum BP, Benzel EC, Mroz TE. Pseudoarthrosis rates in anterior cervical discectomy and fusion: a meta-analysis. Spine J. 15:2016–2027. 2015.
Article21. Song J, Taghavi CE, Hsu DW, Song KJ, Song JH, Lee KB. Radiological changes in anterior cervical discectomy and fusion with cage and plate construct: the significance of the anterior spur formation sign. Spine (Phila Pa 1976). 37:272–279. 2012.
Article22. Song KS, Chaiwat P, Kim HJ, Mesfin A, Park SM, Riew KD. Anterior cervical fusion assessment using reconstructed computed tomographic scans: surgical confirmation of 254 segments. Spine (Phila Pa 1976). 38:2171–2177. 2013.
Article23. Song KS, Piyaskulkaew C, Chuntarapas T, Buchowski JM, Kim HJ, Park MS, et al. Dynamic radiographic criteria for detecting pseudarthrosis following anterior cervical arthrodesis. J Bone Joint Surg Am. 96:557–563. 2014.
Article24. Yoganandan N, Kumaresan S, Pintar FA. Geometric and mechanical properties of human cervical spine ligaments. J Biomech Eng. 122:623–629. 2000.
Article25. Zhang QH, Teo EC, Ng HW, Lee VS. Finite element analysis of moment-rotation relationships for human cervical spine. J Biomech. 39:189–193. 2006.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Review of Finite Element Modeling for Anterior Cervical Discectomy and Fusion
- Diagnosing Pseudoarthrosis After Anterior Cervical Discectomy and Fusion
- Comparing adjacent segment biomechanics between anterior and posterior cervical fusion using patient-specific finite element modeling
- Biomechanical Analysis of Biodegradable Cervical Plates Developed for Anterior Cervical Discectomy and Fusion
- Results of Anterior Cervical Discectomy without Interbody Fusion