Cancer Res Treat.
2017 Oct;49(4):906-914. 10.4143/crt.2016.424.
Genetic Alterations and Their Clinical Implications in High-Recurrence Risk Papillary Thyroid Cancer
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. silkahn@skku.edu
- 2Samsung Biomedical Research Institute, Seoul, Korea.
- 3Department of Pathology, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 4Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2394810
- DOI: http://doi.org/10.4143/crt.2016.424
Abstract
- PURPOSE
Papillary thyroid carcinomas (PTCs) frequently involve genetic alterations. The objective of this study was to investigate genetic alterations and further explore the relationships between these genetic alterations and clinicopathological characteristics in a high-recurrence risk (node positive, N1) PTC group.
MATERIALS AND METHODS
Tumor tissue blocks were obtained from 240 surgically resected patients with histologically confirmed stage III/IV (pT3/4 or N1) PTCs. We screened gene fusions using NanoString's nCounter technology and mutational analysis was performed by direct DNA sequencing. Data describing the clinicopathological characteristics and clinical courses were retrospectively collected.
RESULTS
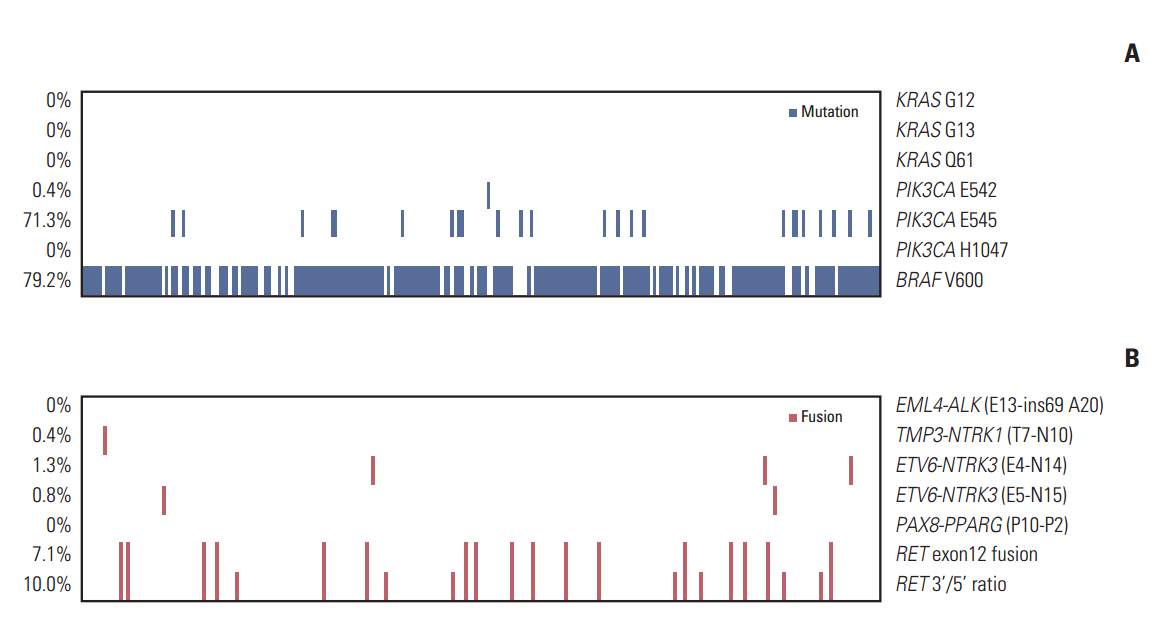
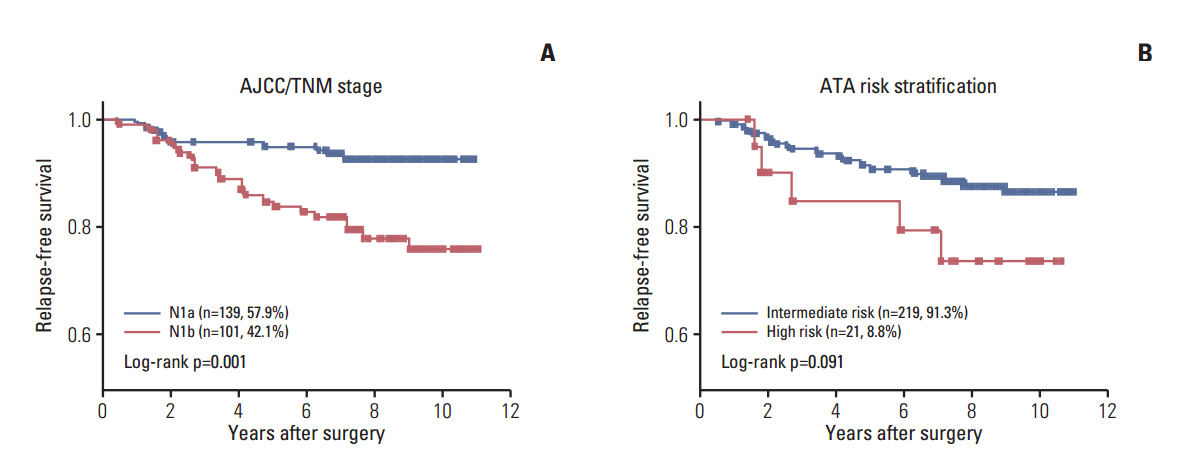
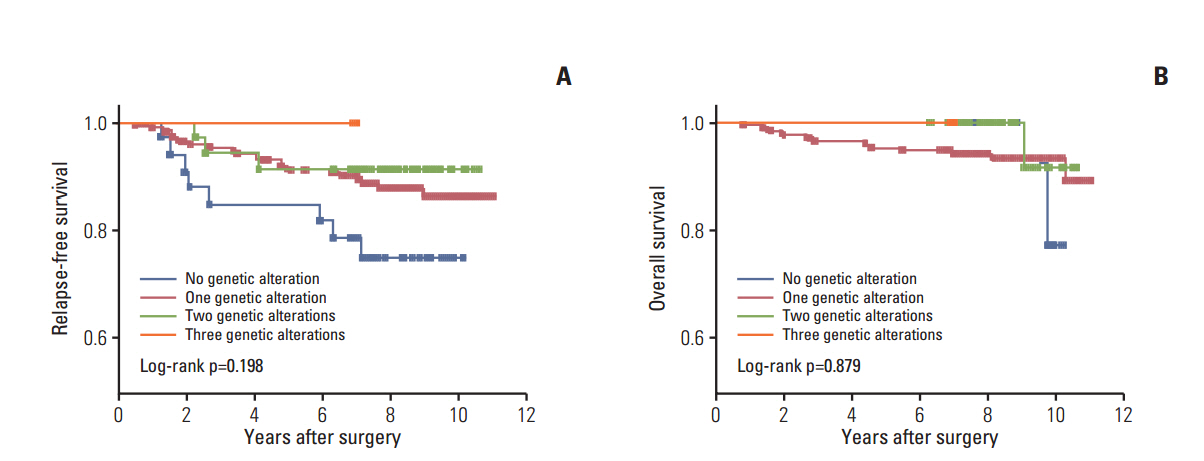
Of the 240 PTC patients, 207 (86.3%) had at least one genetic alteration, including BRAF mutation in 190 patients (79.2%), PIK3CA mutation in 25 patients (10.4%), NTRK1/3 fusion in six patients (2.5%), and RET fusion in 24 patients (10.0%). Concomitant presence of more than two genetic alterations was seen in 36 patients (15%). PTCs harboring BRAF mutation were associated with RET wild-type expression (p=0.001). RET fusion genes have been found to occur with significantly higher frequency in N1b stage patients (p=0.003) or groups of patients aged 45 years or older (p=0.031); however, no significant correlation was found between other genetic alterations. There was no trend toward favorable recurrence-free survival or overall survival among patients lacking genetic alterations.
CONCLUSION
In the selected high-recurrence risk PTC group, most patients had more than one genetic alteration. However, these known alterations could not entirely account for clinicopathological features of high-recurrence risk PTC.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ito Y, Nikiforov YE, Schlumberger M, Vigneri R. Increasing incidence of thyroid cancer: controversies explored. Nat Rev Endocrinol. 2013; 9:178–84.
Article2. Kim WB. A closer look at papillary thyroid carcinoma. Endocrinol Metab (Seoul). 2015; 30:1–6.
Article3. Henderson YC, Shellenberger TD, Williams MD, El-Naggar AK, Fredrick MJ, Cieply KM, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. 2009; 15:485–91.
Article4. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013; 13:184–99.
Article5. Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003; 22:4578–80.
Article6. Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006; 30:216–22.
Article7. Costa AM, Herrero A, Fresno MF, Heymann J, Alvarez JA, Cameselle-Teijeiro J, et al. BRAF mutation associated with other genetic events identifies a subset of aggressive papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2008; 68:618–34.
Article8. Hong AR, Lim JA, Kim TH, Choi HS, Yoo WS, Min HS, et al. The frequency and clinical implications of the BRAF(V600E) mutation in papillary thyroid cancer patients in Korea over the past two decades. Endocrinol Metab (Seoul). 2014; 29:505–13.9. Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK, Lee YJ, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer. 2012; 118:1764–73.10. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013; 309:1493–501.
Article11. Benvenga S, Koch CA. Molecular pathways associated with aggressiveness of papillary thyroid cancer. Curr Genomics. 2014; 15:162–70.
Article12. Song YS, Lim JA, Park YJ. Mutation profile of well-differentiated thyroid cancer in Asians. Endocrinol Metab (Seoul). 2015; 30:252–62.
Article13. Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid. 2010; 20:697–706.
Article14. Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014; 5:4846.
Article15. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–90.16. Greco A, Miranda C, Pierotti MA. Rearrangements of NTRK1 gene in papillary thyroid carcinoma. Mol Cell Endocrinol. 2010; 321:44–9.
Article17. Cho BY, Choi HS, Park YJ, Lim JA, Ahn HY, Lee EK, et al. Changes in the clinicopathological characteristics and outcomes of thyroid cancer in Korea over the past four decades. Thyroid. 2013; 23:797–804.
Article18. Chitikova Z, Pusztaszeri M, Makhlouf AM, Berczy M, Delucinge-Vivier C, Triponez F, et al. Identification of new biomarkers for human papillary thyroid carcinoma employing NanoString analysis. Oncotarget. 2015; 6:10978–93.
Article19. He G, Zhao B, Zhang X, Gong R. Prognostic value of the BRAF V600E mutation in papillary thyroid carcinoma. Oncol Lett. 2014; 7:439–43.
Article20. Cheng SP, Hsu YC, Liu CL, Liu TP, Chien MN, Wang TY, et al. Significance of allelic percentage of BRAF c.1799T > A (V600E) mutation in papillary thyroid carcinoma. Ann Surg Oncol. 2014; 21 Suppl 4:S619–26.21. Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab. 2012; 97:4559–70.22. Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015; 33:42–50.
Article23. Henke LE, Pfeifer JD, Ma C, Perkins SM, DeWees T, El-Mofty S, et al. BRAF mutation is not predictive of long-term outcome in papillary thyroid carcinoma. Cancer Med. 2015; 4:791–9.24. Howell GM, Hodak SP, Yip L. RAS mutations in thyroid cancer. Oncologist. 2013; 18:926–32.
Article25. Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009; 69:4885–93.
Article26. Pennelli G, Vianello F, Barollo S, Pezzani R, Merante Boschin I, Pelizzo MR, et al. BRAF(K601E) mutation in a patient with a follicular thyroid carcinoma. Thyroid. 2011; 21:1393–6.
Article27. Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014; 14:173–86.
Article28. Zou M, Baitei EY, Alzahrani AS, BinHumaid FS, Alkhafaji D, Al-Rijjal RA, et al. Concomitant RAS, RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid. 2014; 24:1256–66.29. Guerra A, Zeppa P, Bifulco M, Vitale M. Concomitant BRAF(V600E) mutation and RET/PTC rearrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid. 2014; 24:254–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetic Alterations in Follicular Cell-derived Thyroid Carcinomas
- Thyroid Lobectomy as an Initial Treatment Option on 1-4 cm Papillary Cancer
- Significance of Lymphovascular Invasion as a Prognostic Factor in Patients with Papillary Thyroid Cancer: a Systematic Review and Meta-Analysis
- Recent Progress of Genome Study for Anaplastic Thyroid Cancer
- Genetic Analysis of Locally Advanced Well-Differentiated Thyroid Cancer: Clinical Prognostic Implications