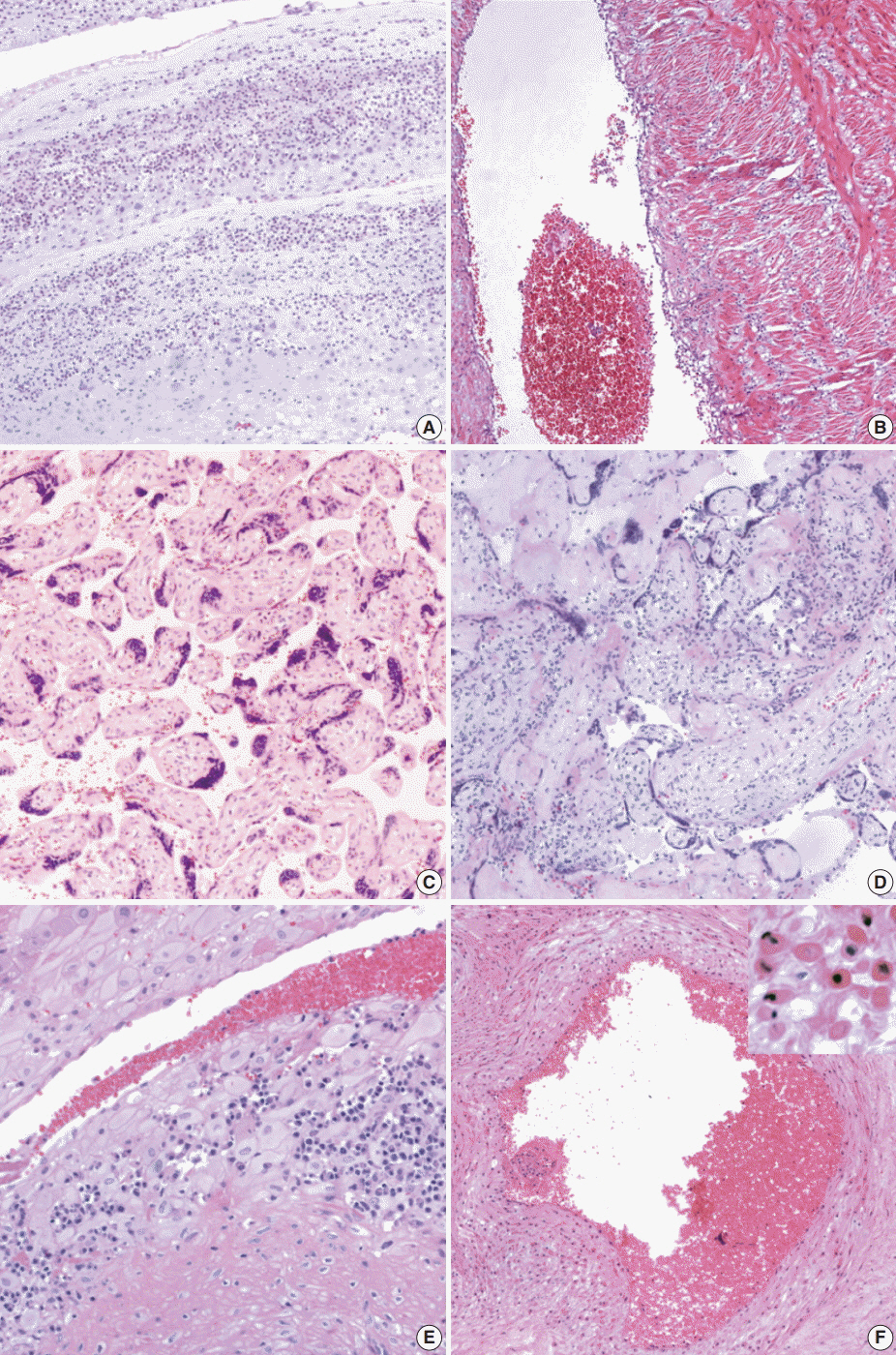
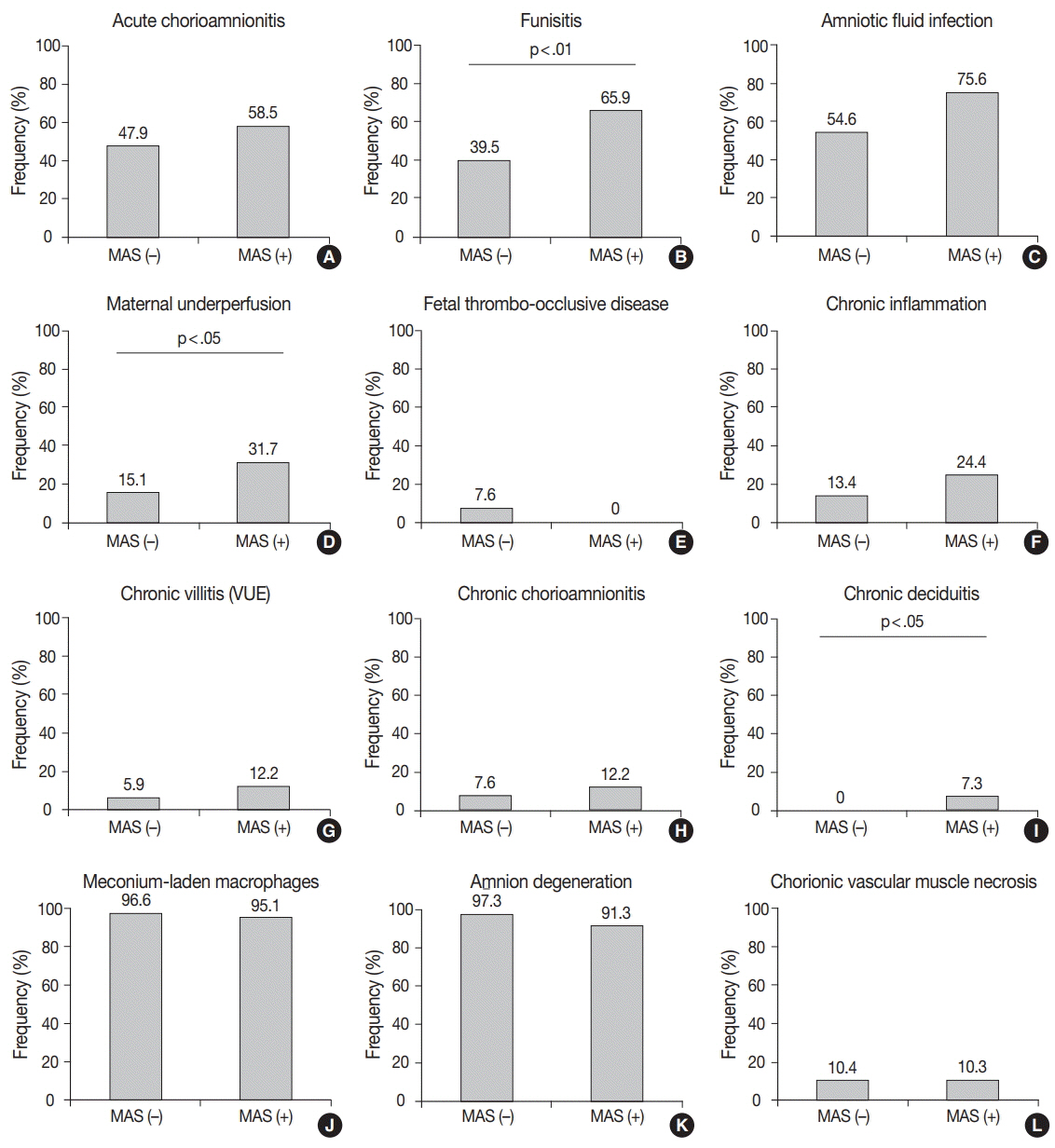
J Pathol Transl Med.
2017 Sep;51(5):488-498. 10.4132/jptm.2017.07.20.
Placental Lesions in Meconium Aspiration Syndrome
- Affiliations
-
- 1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jsunkim@skku.edu
- 2Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Health Sciences and Technology, Sungkyunkwan University, SAIHST, Seoul, Korea.
- KMID: 2392568
- DOI: http://doi.org/10.4132/jptm.2017.07.20
Abstract
- BACKGROUND
Meconium aspiration syndrome (MAS) is defined by respiratory distress requiring supplemental oxygen in a meconium-stained neonate. MAS is clinically subclassified as mild, moderate, and severe according to the oxygen requirement. The aims of this study were to compare the histological findings in the placentas of MAS neonates with those of meconium-stained but non-MAS neonates and to analyze the correlation between the severity of MAS and the grade of its histological parameters.
METHODS
We collected 160 singleton term placentas from neonates with meconium staining at birth from a tertiary medical center, Seoul, Republic of Korea. We reviewed hematoxylin and eosin sections of tissue samples (full-thickness placental disc, chorioamniotic membranes, and umbilical cord).
RESULTS
Funisitis was present more frequently in MAS than in non-MAS (p < .01), of which the stage was correlated with the severity of MAS (p < .001). The histological findings consistent with maternal underperfusion and chronic deciduitis were more frequent in MAS than in non-MAS (p < .05). There was a correlation between the degree of chorionic vascular muscle necrosis and the severity of MAS (p < .05).
CONCLUSIONS
Our results suggest that fetal inflammatory response evidenced by funisitis occurs prenatally in MAS and that the stage of funisitis and of chorionic vascular muscle necrosis may be a predictive marker of the severity of MAS.
Keyword
MeSH Terms
Figure
Reference
-
1. Fischer C, Rybakowski C, Ferdynus C, Sagot P, Gouyon JB. A population-based study of meconium aspiration syndrome in neonates born between 37 and 43 weeks of gestation. Int J Pediatr. 2012; 2012:321545.
Article2. Ahanya SN, Lakshmanan J, Morgan BL, Ross MG. Meconium passage in utero: mechanisms, consequences, and management. Obstet Gynecol Surv. 2005; 60:45–56.3. Hofmeyr GJ, Xu H. Amnioinfusion for meconium-stained liquor in labour. Cochrane Database Syst Rev. 2010; (1):CD000014.
Article4. Lauzon L, Hodnett E. Labour assessment programs to delay admission to labour wards. Cochrane Database Syst Rev. 2001; (3):CD000936.
Article5. Ghidini A, Spong CY. Severe meconium aspiration syndrome is not caused by aspiration of meconium. Am J Obstet Gynecol. 2001; 185:931–8.
Article6. Wiswell TE, Bent RC. Meconium staining and the meconium aspiration syndrome. Unresolved issues. Pediatr Clin North Am. 1993; 40:955–81.
Article7. Choi W, Jeong H, Choi SJ, et al. Risk factors differentiating mild/moderate from severe meconium aspiration syndrome in meconium-stained neonates. Obstet Gynecol Sci. 2015; 58:24–31.
Article8. Hernández C, Little BB, Dax JS, Gilstrap LC 3rd, Rosenfeld CR. Prediction of the severity of meconium aspiration syndrome. Am J Obstet Gynecol. 1993; 169:61–70.
Article9. Spong CY, Ogundipe OA, Ross MG. Prophylactic amnioinfusion for meconium-stained amniotic fluid. Am J Obstet Gynecol. 1994; 171:931–5.
Article10. Cornish JD, Dreyer GL, Snyder GE, et al. Failure of acute perinatal asphyxia or meconium aspiration to produce persistent pulmonary hypertension in a neonatal baboon model. Am J Obstet Gynecol. 1994; 171:43–9.
Article11. Jovanovic R, Nguyen HT. Experimental meconium aspiration in guinea pigs. Obstet Gynecol. 1989; 73:652–6.
Article12. Redline RW, Faye-Petersen O, Heller D, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003; 6:435–48.
Article13. Redline RW, Boyd T, Campbell V, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004; 7:237–49.
Article14. Redline RW, Ariel I, Baergen RN, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004; 7:443–52.
Article15. Altshuler G, Arizawa M, Molnar-Nadasdy G. Meconium-induced umbilical cord vascular necrosis and ulceration: a potential link between the placenta and poor pregnancy outcome. Obstet Gynecol. 1992; 79(5 Pt 1):760–6.16. Miller PW, Coen RW, Benirschke K. Dating the time interval from meconium passage to birth. Obstet Gynecol. 1985; 66:459–62.17. Burgess AM, Hutchins GM. Inflammation of the lungs, umbilical cord and placenta associated with meconium passage in utero: review of 123 autopsied cases. Pathol Res Pract. 1996; 192:1121–8.18. Espinheira MC, Grilo M, Rocha G, Guedes B, Guimarães H. Meconium aspiration syndrome: the experience of a tertiary center. Rev Port Pneumol. 2011; 17:71–6.19. Lee J, Romero R, Lee KA, et al. Meconium aspiration syndrome: a role for fetal systemic inflammation. Am J Obstet Gynecol. 2016; 214:366. e1-9.
Article20. Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010; 23:1000–11.
Article21. Khong TY, Bendon RW, Qureshi F, et al. Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol. 2000; 31:292–5.22. Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015; 213(4 Suppl):S29–52.
Article23. Overbach AM, Daniel SJ, Cassady G. The value of umbilical cord histology in the management of potential perinatal infection. J Pediatr. 1970; 76:22–31.
Article24. Maudsley RF, Brix GA, Hinton NA, Robertson EM, Bryans AM, Haust MD. Placental inflammation and infection: a prospective bacteriologic and histologic study. Am J Obstet Gynecol. 1966; 95:648–59.25. D’Alquen D, Kramer BW, Seidenspinner S, et al. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res. 2005; 57:263–9.
Article26. Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002; 11:18–25.
Article27. Naccasha N, Hinson R, Montag A, Ismail M, Bentz L, Mittendorf R. Association between funisitis and elevated interleukin-6 in cord blood. Obstet Gynecol. 2001; 97:220–4.
Article28. Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000; 183:1124–9.
Article29. Romero R, Hanaoka S, Mazor M, et al. Meconium-stained amniotic fluid: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1991; 164:859–62.
Article30. Mazor M, Furman B, Wiznitzer A, Shoham-Vardi I, Cohen J, Ghezzi F. Maternal and perinatal outcome of patients with preterm labor and meconium-stained amniotic fluid. Obstet Gynecol. 1995; 86:830–3.
Article31. Halliday HL, Hirata T. Perinatal listeriosis: a review of twelve patients. Am J Obstet Gynecol. 1979; 133:405–10.32. Cassell GH, Davis RO, Waites KB, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16-20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983; 10(4 Suppl):294–302.33. Mazor M, Froimovich M, Lazer S, Maymon E, Glezerman M. Listeria monocytogenes: the role of transabdominal amniocentesis in febrile patients with preterm labor. Arch Gynecol Obstet. 1992; 252:109–12.34. Mazor M, Hershkovitz R, Bashiri A, et al. Meconium stained amniotic fluid in preterm delivery is an independent risk factor for perinatal complications. Eur J Obstet Gynecol Reprod Biol. 1998; 81:9–13.
Article35. Jessop FA, Lees CC, Pathak S, Hook CE, Sebire NJ. Funisitis is associated with adverse neonatal outcome in low-risk unselected deliveries at or near term. Virchows Arch. 2016; 468:503–7.
Article36. Du H, Liu E, Xu C, Zhao S, Xiang H, Li Z. Prognostic value of funisitis and/or chorionic vasculitis compared to histologic chorioamnionitis in full-term infants. J Matern Fetal Neonatal Med. 2017; 30:169–73.
Article37. Kim CJ, Yoon BH, Romero R, et al. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. Am J Obstet Gynecol. 2001; 185:496–500.
Article38. Matsuda T, Nakajima T, Hattori S, et al. Necrotizing funisitis: clinical significance and association with chronic lung disease in premature infants. Am J Obstet Gynecol. 1997; 177:1402–7.
Article39. DiSalvo D. The correlation between placental pathology and intraventricular hemorrhage in the preterm infant. The Developmental Epidemiology Network Investigators. Pediatr Res. 1998; 43:15–9.40. Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000; 182:675–81.
Article41. Leviton A, Paneth N, Reuss ML, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res. 1999; 46:566–75.42. Mittendorf R, Montag AG, MacMillan W, et al. Components of the systemic fetal inflammatory response syndrome as predictors of impaired neurologic outcomes in children. Am J Obstet Gynecol. 2003; 188:1438–46.
Article43. Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol. 2010; 116(2 Pt 1):387–92.44. Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996; 97:210–5.
Article45. Moscuzza F, Belcari F, Nardini V, et al. Correlation between placental histopathology and fetal/neonatal outcome: chorioamnionitis and funisitis are associated to intraventricular haemorrage and retinopathy of prematurity in preterm newborns. Gynecol Endocrinol. 2011; 27:319–23.
Article46. Tominaga T, Page EW. Accommodation of the human placenta to hypoxia. Am J Obstet Gynecol. 1966; 94:679–91.
Article47. Mayhew TM, Barker BL. Villous trophoblast: morphometric perspectives on growth, differentiation, turnover and deposition of fibrin-type fibrinoid during gestation. Placenta. 2001; 22:628–38.
Article48. Huppertz B, Kingdom J, Caniggia I, et al. Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation. Placenta. 2003; 24:181–90.
Article49. Jackson MR, Walsh AJ, Morrow RJ, Mullen JB, Lye SJ, Ritchie JW. Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: relationship with umbilical artery Doppler waveforms. Am J Obstet Gynecol. 1995; 172(2 Pt 1):518–25.
Article50. Karsdorp VH, Dirks BK, van der Linden JC, van Vugt JM, Baak JP, van Geijn HP. Placenta morphology and absent or reversed end diastolic flow velocities in the umbilical artery: a clinical and morphometrical study. Placenta. 1996; 17:393–9.
Article51. Macara L, Kingdom JC, Kohnen G, Bowman AW, Greer IA, Kaufmann P. Elaboration of stem villous vessels in growth restricted pregnancies with abnormal umbilical artery Doppler waveforms. Br J Obstet Gynaecol. 1995; 102:807–12.
Article52. Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol. 1996; 175:1534–42.
Article53. Madazli R, Somunkiran A, Calay Z, Ilvan S, Aksu MF. Histomorphology of the placenta and the placental bed of growth restricted foetuses and correlation with the Doppler velocimetries of the uterine and umbilical arteries. Placenta. 2003; 24:510–6.
Article54. Kaplan C, Lowell DM, Salafia C. College of American Pathologists Conference XIX on the Examination of the Placenta: report of the Working Group on the Definition of Structural Changes Associated with Abnormal Function in the Maternal/Fetal/Placental Unit in the Second and Third Trimesters. Arch Pathol Lab Med. 1991; 115:709–16.55. Khong TY. The Robertson-Brosens-Dixon hypothesis: evidence for the role of haemochorial placentation in pregnancy success. Br J Obstet Gynaecol. 1991; 98:1195–9.
Article56. Kliman HJ. Uteroplacental blood flow: the story of decidualization, menstruation, and trophoblast invasion. Am J Pathol. 2000; 157:1759–68.57. Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986; 93:1049–59.
Article58. Barth WH Jr, Genest DR, Riley LE, Frigoletto FD Jr, Benacerraf BR, Greene MF. Uterine arcuate artery Doppler and decidual microvascular pathology in pregnancies complicated by type I diabetes mellitus. Ultrasound Obstet Gynecol. 1996; 8:98–103.
Article59. Thureen PJ, Hall DM, Hoffenberg A, Tyson RW. Fatal meconium aspiration in spite of appropriate perinatal airway management: pulmonary and placental evidence of prenatal disease. Am J Obstet Gynecol. 1997; 176:967–75.
Article60. De Wolf F, Brosens I, Renaer M. Fetal growth retardation and the maternal arterial supply of the human placenta in the absence of sustained hypertension. Br J Obstet Gynaecol. 1980; 87:678–85.61. Lackman F, Capewell V, Richardson B, daSilva O, Gagnon R. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol. 2001; 184:946–53.
Article62. Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol. 1997; 89:265–71.
Article63. Naeye RL. Pregnancy hypertension, placental evidences of low uteroplacental blood flow, and spontaneous premature delivery. Hum Pathol. 1989; 20:441–4.
Article64. Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. JAMA. 2002; 287:3183–6.
Article65. Edmonds P. An introduction to meconium. Midwifery Today Int Midwife. 2014; (111):32–3.66. Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015; 213(4 Suppl):S53–69.
Article67. Naeye RL. Functionally important disorders of the placenta, umbilical cord, and fetal membranes. Hum Pathol. 1987; 18:680–91.
Article68. Katzman PJ. Chronic inflammatory lesions of the placenta. Semin Perinatol. 2015; 39:20–6.
Article69. King EL, Redline RW, Smith SD, Kraus FT, Sadovsky Y, Nelson DM. Myocytes of chorionic vessels from placentas with meconium-associated vascular necrosis exhibit apoptotic markers. Hum Pathol. 2004; 35:412–7.
Article70. Cimic A, Baergen RN. Meconium-associated umbilical vascular myonecrosis: correlations with adverse outcome and placental pathology. Pediatr Dev Pathol. 2016; 19:315–9.
Article71. Müller H, Weiss C, Renner M, Felderhoff-Müser U, Mollenhauer J. DMBT1 promotes basal and meconium-induced nitric oxide production in human lung epithelial cells in vitro. Histochem Cell Biol. 2017; 147:389–97.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical observation of meconium aspiration syndrome: prognostic implication of early meconium suctioning
- A Study of Meconium Aspiration Syndrome
- A clinical study on meconium-stained babies
- A Clinical Observation of Meconium Aspiration Syndrome
- Diagnosis of Meconium Aspiration by Spectrophotometric Analysis of Urine