J Vet Sci.
2012 Dec;13(4):413-417.
Suitability of autologous serum for expanding rabbit adipose-derived stem cell populations
- Affiliations
-
- 1Department of Neurology, Biomedical Research Institute, Seoul National University Hospital, Seoul 110-744, Korea.
- 2Department of Orthopedic Surgery, Seoul National University Hospital, Seoul 110-744, Korea. drjacobkim@gmail.com
Abstract
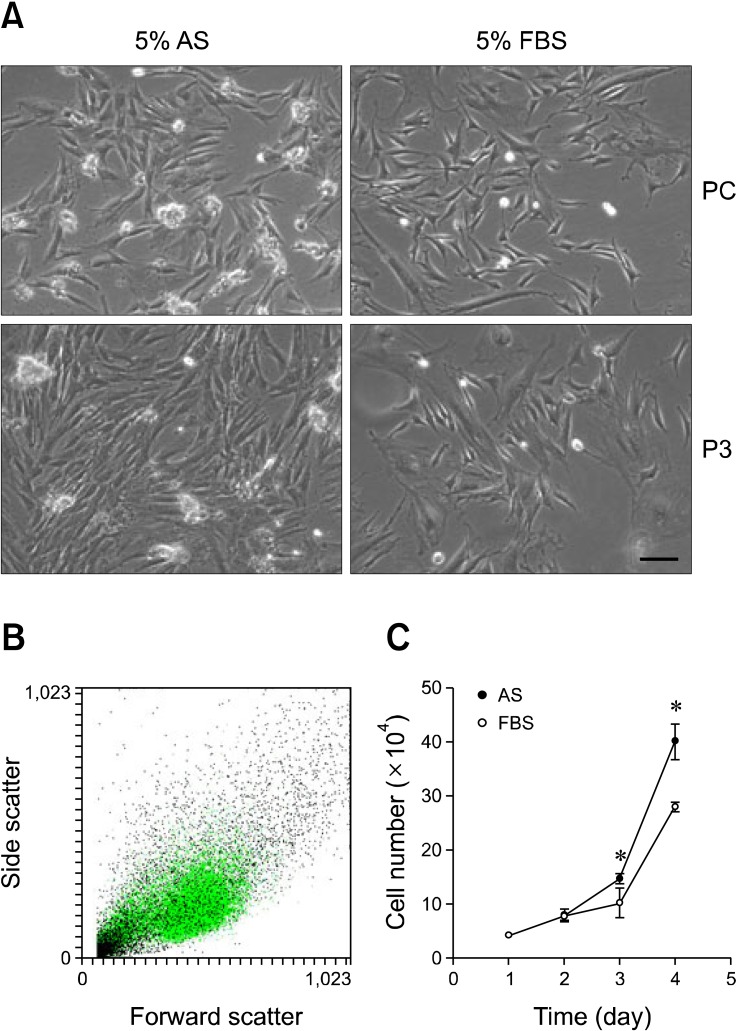
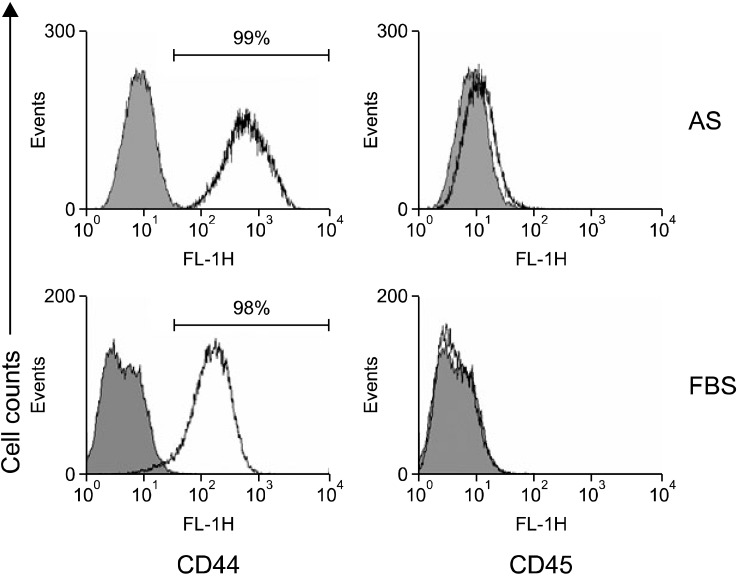
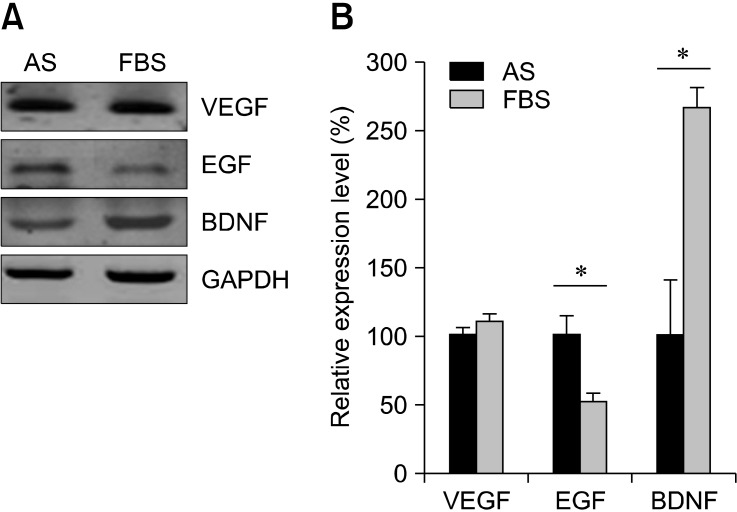
- Adipose-derived stem cells (ASCs) are believed to have potential use for treating many illnesses. Most cells, including ASCs, are generally cultured in medium containing fetal bovine serum (FBS). However, FBS, which could induce an immune response or infection, is not recommended for clinical applications. In the present study, we evaluated the morphology, proliferation rate, and characterization of rabbit ASCs grown in medium containing autologous serum (AS) and compared these cells to ones cultured with FBS. Morphological changes were monitored by microscopy and flow cytometry. Proliferation rates were assessed with cell counting and ASC phenotypes were characterized by flow cytometry using representative surface markers (CD44 and CD45). Expression of epidermal growth factor, brain-derived neurotrophic factor, and vascular endothelial growth factor was measured by reverse transcription-polymerase chain reaction. Results of our study showed that ASCs had a greater expansion rate in AS without developing morphological heterogeneity than cells grown in FBS. AS-cultured ASCs expressed representative growth factors, CD44 but not CD45, similar to cells cultured in FBS. Expression levels of some growth factors were different between AS and FBS. In conclusion, our findings indicated that AS could potentially be used as a culture medium supplement for the expansion of autologous ASCs.
MeSH Terms
Figure
Reference
-
1. Chen L, Tredget EE, Wu PYG, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008; 3:e1886. PMID: 18382669.
Article2. Chung JY, Kim W, Im W, Yoo DY, Choi JH, Hwang IK, Won MH, Chang IB, Cho BM, Hwang HS, Moon SM. Neuroprotective effects of adipose-derived stem cells against ischemic neuronal damage in the rabbit spinal cord. J Neurol Sci. 2012; 317:40–46. PMID: 22475376.
Article3. de Girolamo L, Arrigoni E, Stanco D, Lopa S, Di Giancamillo A, Addis A, Borgonovo S, Dellavia C, Domeneghini C, Brini AT. Role of autologous rabbit adipose-derived stem cells in the early phases of the repairing process of critical bone defects. J Orthop Res. 2011; 29:100–108. PMID: 20607837.
Article4. De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, Benhaim P, Hedrick MH, Fraser JK. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003; 89:267–270. PMID: 14556988.
Article5. Drewa T, Biliński PJ, Gałazka P, Czaplewski A, Drewa Ł, Woźniak A, Sir J. Influence of autologous serum on rabbit chondrocytes proliferation in vitro. Comparing study to standard sera supplements. Ortop Traumatol Rehabil. 2003; 5:751–757. PMID: 18034068.6. Ebrahimian TG, Pouzoulet F, Squiban C, Buard V, André M, Cousin B, Gourmelon P, Benderitter M, Casteilla L, Tamarat R. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol. 2009; 29:503–510. PMID: 19201690.
Article7. Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010; 5:1294–1311. PMID: 20595958.
Article8. Froehlich H, Gulati R, Boilson B, Witt T, Harbuzariu A, Kleppe L, Dietz AB, Lerman A, Simari RD. Carotid repair using autologous adipose-derived endothelial cells. Stroke. 2009; 40:1886–1891. PMID: 19286583.
Article9. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008; 103:1204–1219. PMID: 19028920.
Article10. Honda A, Hirose M, Hatori M, Matoba S, Miyoshi H, Inoue K, Ogura A. Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine. J Biol Chem. 2010; 285:31362–31369. PMID: 20670936.11. Im W, Chung JY, Kim SH, Kim M. Efficacy of autologous serum in human adipose-derived stem cells; cell markers, growth factors and differentiation. Cell Mol Biol (Noisy-le-grand). 2011; 57(Suppl):OL1470–OL1475. PMID: 21396339.12. Johnson LF, deSerres S, Herzog SR, Peterson HD, Meyer AA. Antigenic cross-reactivity between media supplements for cultured keratinocyte grafts. J Burn Care Rehabil. 1991; 12:306–312. PMID: 1939301.
Article13. Klein R, Dumble LJ. Transmission of Creutzfeldt-Jakob disease by blood transfusion. Lancet. 1993; 341:768. PMID: 8095678.
Article14. Lee ST, Chu K, Jung KH, Im WS, Park JE, Lim HC, Won CH, Shin SH, Lee SK, Kim M, Roh JK. Slowed progression in models of Huntington disease by adipose stem cell transplantation. Ann Neurol. 2009; 66:671–681. PMID: 19938161.
Article15. Lindroos B, Boucher S, Chase L, Kuokkanen H, Huhtala H, Haataja R, Vemuri M, Suuronen R, Miettinen S. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009; 11:958–972. PMID: 19903107.
Article16. Mackensen A, Dräger R, Schlesier M, Mertelsmann R, Lindemann A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2000; 49:152–156. PMID: 10881694.
Article17. Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006; 7:395–406. PMID: 16760919.
Article18. Mazlyzam AL, Aminuddin BS, Saim L, Ruszymah BHI. Human serum is an advantageous supplement for human dermal fibroblast expansion: clinical implications for tissue engineering of skin. Arch Med Res. 2008; 39:743–752. PMID: 18996287.
Article19. Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005; 25:2542–2547. PMID: 16224047.
Article20. Rada T, Reis RL, Gomes ME. Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev. 2011; 7:64–76. PMID: 20396979.
Article21. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004; 109:1292–1298. PMID: 14993122.
Article22. Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B, Villageois A, Bagnis C, Breittmayer JP, Groux H, Ailhaud G, Dani C. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005; 201:1397–1405. PMID: 15867092.
Article23. Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, Drexler C, Lanzer G, Linkesch W, Strunk D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007; 47:1436–1446. PMID: 17655588.
Article24. Shafaei H, Esmaeili A, Mardani M, Razavi S, Hashemibeni B, Nasr-Esfahani MH, Shiran MB, Esfandiari E. Effects of human placental serum on proliferation and morphology of human adipose tissue-derived stem cells. Bone Marrow Transplant. 2011; 46:1464–1471. PMID: 21217787.
Article25. Tuschong L, Soenen SL, Blaese RM, Candotti F, Muul LM. Immune response to fetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum Gene Ther. 2002; 13:1605–1610. PMID: 12228015.
Article26. Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith PG. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996; 347:921–925. PMID: 8598754.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipose-derived stem cells: characterization and clinical application
- Differentiation of adipose-derived stem cells into Schwann-like cells: fetal bovine serum or human serum?
- Fat grafts enriched with adipose-derived stem cells
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- Familial Occurrence of Pulmonary Embolism after Intravenous, Adipose Tissue-Derived Stem Cell Therapy