Allergy Asthma Immunol Res.
2016 May;8(3):257-263. 10.4168/aair.2016.8.3.257.
Induction of Bronchial Tolerance After 1 Cycle of Monophosphoryl-A-Adjuvanted Specific Immunotherapy in Children With Grass Pollen Allergies
- Affiliations
-
- 1Children's Hospital, Department of Allergy, Pneumology and Cystic Fibrosis, Goethe-University, Frankfurt/Main, Germany. Martin.Rosewich@kgu.de
- KMID: 2391046
- DOI: http://doi.org/10.4168/aair.2016.8.3.257
Abstract
- PURPOSE
Subcutaneous allergen-specific immunotherapy (SCIT) is a well-established and clinically effective method to treat allergic diseases, such as rhinitis and asthma. It remains unclear how soon after initiation of an ultra-short course of grass pollen immunotherapy adjuvanted with monophosphoryl lipid A (MPL)-specific bronchial tolerance can be induced.
METHODS
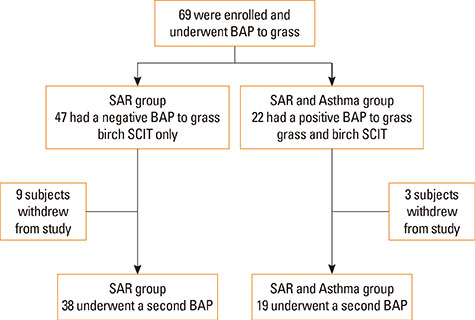
In a prospective study of 69 children double-sensitized to birch and grass pollens (51 males, average age 11.1 years), development of bronchial tolerance after 1 cycle of SCIT for grass was evaluated. In all the patients, the bronchial allergen provocation test (BAP) was performed before and after treatment. According to the results of the first BAP, the patients were divided into 2 groups: those showing a negative BAP with a decrease in FEV1 of <20% (seasonal allergic rhinitis [SAR] group, n=47); and those showing a positive BAP with a decrease in FEV1 of > or =20% (SAR with allergic asthma [SAR and Asthma] group, n=22). All the patients received MPL-adjuvanted, ultra-short course immunotherapy for birch, but only those with a positive BAP to grass received MPL-SCIT for grass.
RESULTS
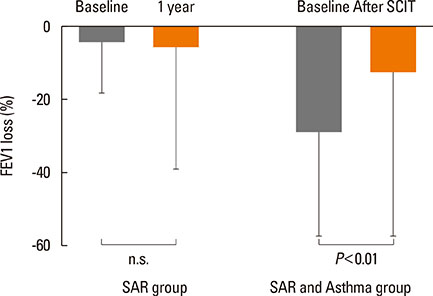
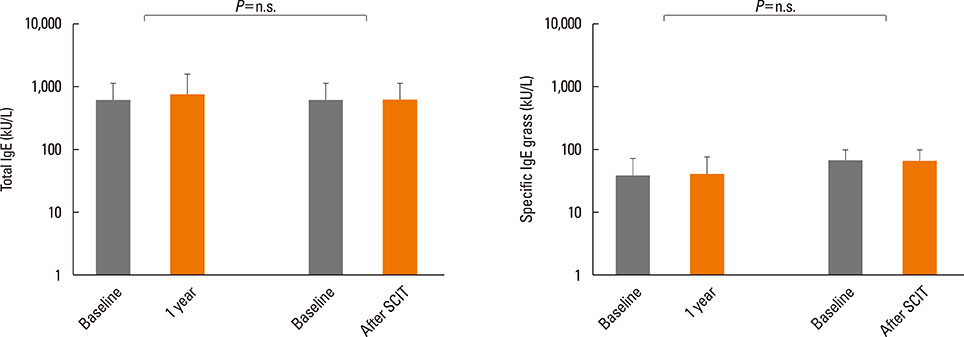
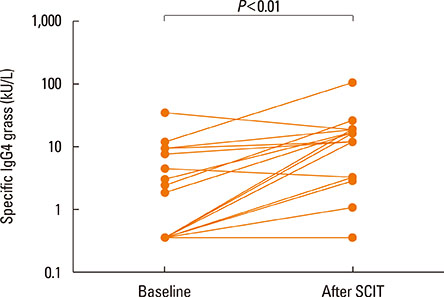
After the pollen season, the BAP in the SAR group remained unchanged, while it was improved in the SAR and Asthma group (decrease in FEV1 of 28.8% vs 12.5%, P<0.01). The IgG4 levels increased after SCIT (median before SCIT 0.34 to 11.4 after SCIT), whereas the total and specific IgE levels remained unchanged.
CONCLUSIONS
After 1 cycle of MPL-SCIT, specific bronchial tolerance may be significantly induced, whereas in patients without SCIT, bronchial hyperactivity may remain unchanged.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Novel strategies in immunotherapy for allergic diseases
Mohana Rajakulendran, Elizabeth Huiwen Tham, Jian Yi Soh, HP Van Bever
Asia Pac Allergy. 2018;8(2):e14. doi: 10.5415/apallergy.2018.8.e14.Novel strategies in immunotherapy for allergic diseases
Mohana Rajakulendran, Elizabeth Huiwen Tham, Jian Yi Soh, HP Van Bever
Asia Pac Allergy. 2018;8(2):. doi: 10.5415/apallergy.2018.8.e14.
Reference
-
1. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006; 355:2226–2235.2. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–1296.e3.3. Lee J, Park CO, Lee KH. Specific immunotherapy in atopic dermatitis. Allergy Asthma Immunol Res. 2015; 7:221–229.4. Ross RN, Nelson HS, Finegold I. Effectiveness of specific immunotherapy in the treatment of hymenoptera venom hypersensitivity: a meta-analysis. Clin Ther. 2000; 22:351–358.5. Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001; 31:1392–1397.6. Kim ST. Outcome of sublingual immunotherapy in patients with allergic rhinitis sensitive to house dust mites. Allergy Asthma Immunol Res. 2015; 7:99–100.7. Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001; 56:498–505.8. Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A. Study Group. Efficacy and safety of preseasonal-specific immunotherapy with an aluminium-adsorbed six-grass pollen allergoid. Allergy. 2005; 60:801–807.9. Canonica GW, Baena-Cagnani CE, Bousquet J, Bousquet PJ, Lockey RF, Malling HJ, et al. Recommendations for standardization of clinical trials with allergen specific immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007; 62:317–324.10. Shamji MH, James LK, Durham SR. Serum immunologic markers for monitoring allergen-specific immunotherapy. Immunol Allergy Clin North Am. 2011; 31:311–323. x11. Rosewich M, Schulze J, Eickmeier O, Telles T, Rose MA, Schubert R, et al. Tolerance induction after specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A in children. Clin Exp Immunol. 2010; 160:403–410.12. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010; CD001186.13. Schulze J, Rosewich M, Dressler M, Riemer C, Rose MA, Zielen S. Bronchial allergen challenge using the medicaid dosimeter. Int Arch Allergy Immunol. 2012; 157:89–97.14. Rosewich M, Arendt S, El Moussaoui S, Schulze J, Schubert R, Zielen S. Bronchial allergen provocation: a useful method to assess the efficacy of specific immunotherapy in children. Pediatr Allergy Immunol. 2013; 24:434–440.15. Rosewich M, Lee D, Zielen S. Pollinex Quattro: an innovative four injections immunotherapy in allergic rhinitis. Hum Vaccin Immunother. 2013; 9:1523–1531.16. Rosewich M, Rose MA, Eickmeier O, Travaci M, Kitz R, Zielen S. Montelukast as add-on therapy to beta-agonists and late airway response. Eur Respir J. 2007; 30:56–61.17. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.18. Schulze J, Reinmüller W, Herrmann E, Rosewich M, Rose MA, Zielen S. Bronchial allergen challenges in children - safety and predictors. Pediatr Allergy Immunol. 2013; 24:19–27.19. Kleine-Tebbe J, Walmar M, Bitsch-Jensen K, Decot E, Pfaar O, de Rojas DH, et al. Negative clinical results from a randomised, double-blind, placebo-controlled trial evaluating the efficacy of two doses of immunologically enhanced, grass subcutaneous immunotherapy despite dose-dependent immunological response. Clin Drug Investig. 2014; 34:577–586.20. Kuehr J, Brauburger J, Zielen S, Schauer U, Kamin W, Von Berg A, et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002; 109:274–280.21. Patel P, Holdich T, Fischer von, Huber B. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J Allergy Clin Immunol. 2014; 133:121–129.e1-2.22. Wechsler ME, Kelley JM, Boyd IO, Dutile S, Marigowda G, Kirsch I, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011; 365:119–126.23. Horak F, Toth J, Hirschwehr R, Marks B, Stübner UP, Jäger S, et al. Effect of continuous allergen challenge on clinical symptoms and mediator release in dust-mite-allergic patients. Allergy. 1998; 53:68–72.24. Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007; 62:943–948.25. Eng PA, Reinhold M, Gnehm HP. Long-term efficacy of preseasonal grass pollen immunotherapy in children. Allergy. 2002; 57:306–312.26. Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999; 341:468–475.27. Frew AJ, Powell RJ, Corrigan CJ, Durham SR. UK Immunotherapy Study Group. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006; 117:319–325.28. Tworek D, Bochenska-Marciniak M, Kuprys-Lipinska I, Kupczyk M, Kuna P. Perennial is more effective than preseasonal subcutaneous immunotherapy in the treatment of seasonal allergic rhinoconjunctivitis. Am J Rhinol Allergy. 2013; 27:304–308.29. Stelmach I, Sobocińska A, Majak P, Smejda K, Jerzyńska J, Stelmach W. Comparison of the long-term efficacy of 3- and 5-year house dust mite allergen immunotherapy. Ann Allergy Asthma Immunol. 2012; 109:274–278.30. Tabar AI, Arroabarren E, Echechipía S, García BE, Martin S, Alvarez-Puebla MJ. Three years of specific immunotherapy may be sufficient in house dust mite respiratory allergy. The J Allergy Clin Immunol. 2011; 127:57–63. 63.e1–63.e3.31. Marogna M, Tomassetti D, Bernasconi A, Colombo F, Massolo A, Businco AD, et al. Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol. 2008; 101:206–211.32. Novembre E, Galli E, Landi F, Caffarelli C, Pifferi M, De Marco E, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004; 114:851–857.33. Jacobsen L, Wahn U, Bilo MB. Allergen-specific immunotherapy provides immediate, long-term and preventive clinical effects in children and adults: the effects of immunotherapy can be categorised by level of benefit -the centenary of allergen specific subcutaneous immunotherapy. Clin Transl Allergy. 2012; 2:8.34. Madonini E, Agostinis F, Barra R, Berra A, Donadio D, Pappacoda A, et al. Long-term and preventive effects of sublingual allergen-specific immunotherapy: a retrospective, multicentric study. Int J Immunopathol Pharmacol. 2003; 16:73–79.35. Compalati E, Braido F, Canonica GW. An update on allergen immunotherapy and asthma. Curr Opin Pulm Med. 2014; 20:109–117.36. Gerhold K, Bluemchen K, Franke A, Stock P, Hamelmann E. Exposure to endotoxin and allergen in early life and its effect on allergen sensitization in mice. J Allergy Clin Immunol. 2003; 112:389–396.37. Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol. 2014; 133:551–559.