Chonnam Med J.
2017 Sep;53(3):178-186. 10.4068/cmj.2017.53.3.178.
Role of Recepteur D'origine Nantais on Gastric Cancer Development and Progression
- Affiliations
-
- 1Department of Biochemistry, Chonnam National University Medical School, Gwangju, Korea. ydjung@chonnam.ac.kr
- KMID: 2390431
- DOI: http://doi.org/10.4068/cmj.2017.53.3.178
Abstract
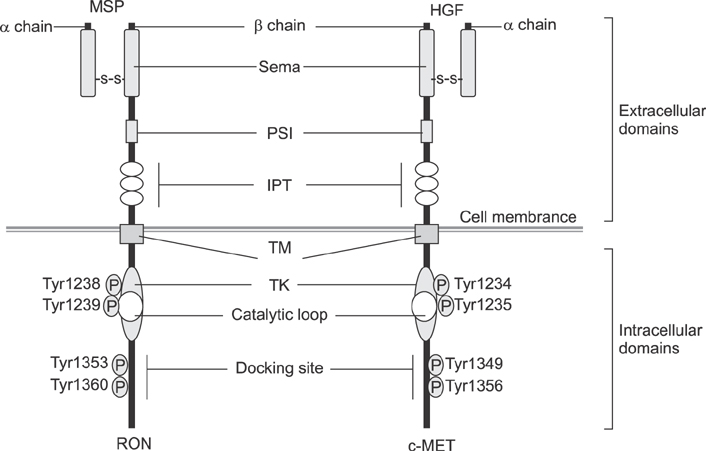
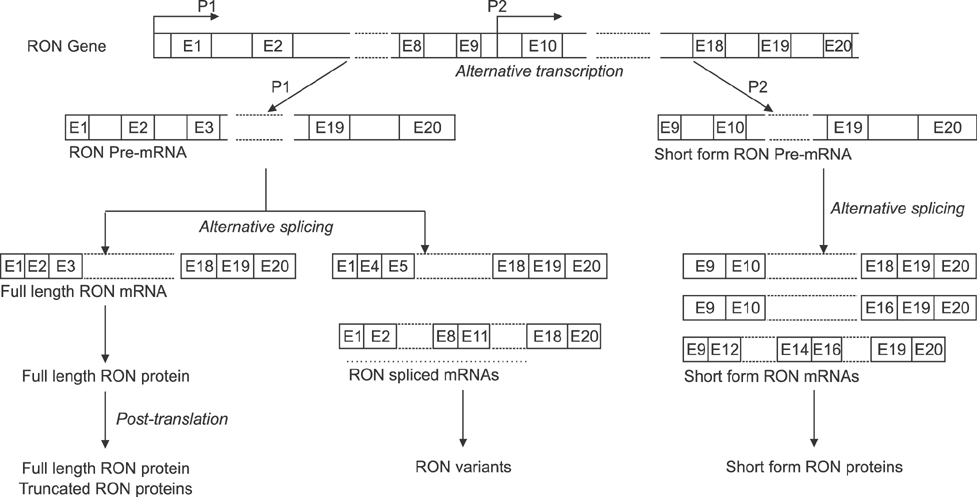
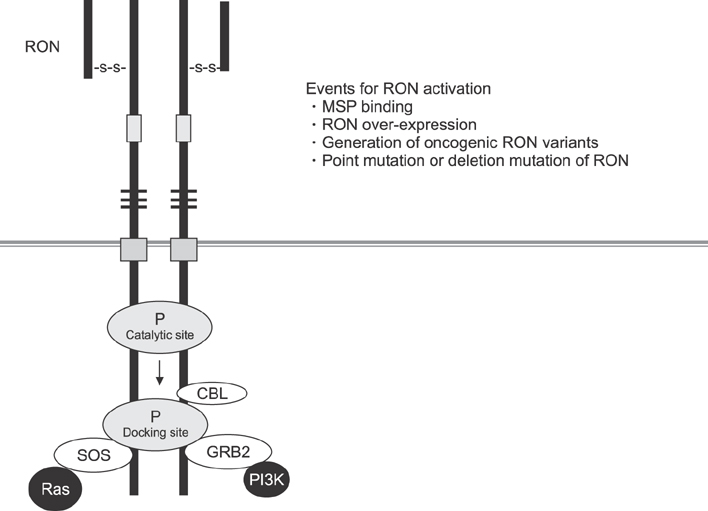
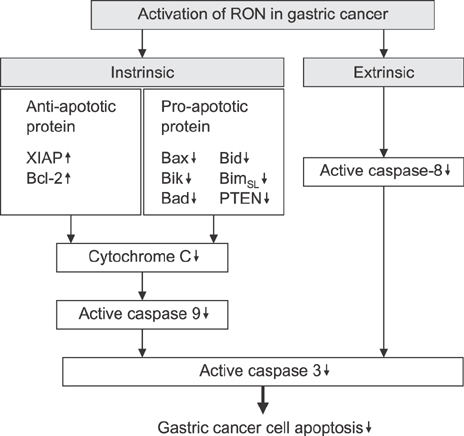
- Recepteur d'origine nantais (RON) is a receptor tyrosine kinase belonging to the subfamily of which c-MET is the prototype. Large epidemiologic studies have confirmed the strong association between RON and gastric cancer development. Constitutive activation of RON signaling directly correlates with tumorigenic phenotypes of gastric cancer and a poor survival rate in advanced gastric cancer patients. In this review, we focus on recent evidence of the aberrant expression and activation of RON in gastric cancer tumors and provide insights into the mechanism of RON signaling associated with gastric cancer progression and metastasis. Current therapeutics against RON in gastric cancer are summarized.
MeSH Terms
Figure
Reference
-
1. Ronsin C, Muscatelli F, Mattei MG, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993; 8:1195–1202.2. Gaudino G, Follenzi A, Naldini L, Collesi C, Santoro M, Gallo KA, et al. RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 1994; 13:3524–3532.
Article3. Zarei O, Benvenuti S, Ustun-Alkan F, Hamzeh-Mivehroud M, Dastmalchi S. Strategies of targeting the extracellular domain of RON tyrosine kinase receptor for cancer therapy and drug delivery. J Cancer Res Clin Oncol. 2016; 142:2429–2446.
Article4. Wang MH, Padhye SS, Guin S, Ma Q, Zhou YQ. Potential therapeutics specific to c-MET/RON receptor tyrosine kinases for molecular targeting in cancer therapy. Acta Pharmacol Sin. 2010; 31:1181–1188.
Article5. Quantin B, Schuhbaur B, Gesnel MC, Doll'e P, Breathnach R. Restricted expression of the ron gene encoding the macrophage stimulating protein receptor during mouse development. Dev Dyn. 1995; 204:383–390.
Article6. Del Gatto F, Gilbert E, Ronsin C, Breathnach R. Structure of the promoter for the human macrophage stimulating protein receptor gene. Biochim Biophys Acta. 1995; 1263:93–95.
Article7. Angeloni D, Danilkovitch-Miagkova A, Ivanov SV, Breathnach R, Johnson BE, Leonard EJ, et al. Gene structure of the human receptor tyrosine kinase RON and mutation analysis in lung cancer samples. Genes Chromosomes Cancer. 2000; 29:147–156.
Article8. Bardella C, Costa B, Maggiora P, Patane' S, Olivero M, Ranzani GN, et al. Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell-cell adhesion through E-cadherin transcriptional repression. Cancer Res. 2004; 64:5154–5161.
Article9. Angeloni D, Danilkovitch-Miagkova A, Ivanova T, Braga E, Zabarovsky E, Lerman MI. Hypermethylation of Ron proximal promoter associates with lack of full-length Ron and transcription of oncogenic short-Ron from an internal promoter. Oncogene. 2007; 26:4499–4512.
Article10. Collesi C, Santoro MM, Gaudino G, Comoglio PM. A splicing variant of the RON transcript induces constitutive tyrosine kinase activity and an invasive phenotype. Mol Cell Biol. 1996; 16:5518–5526.
Article11. Santoro MM, Collesi C, Grisendi S, Gaudino G, Comoglio PM. Constitutive activation of the RON gene promotes invasive growth but not transformation. Mol Cell Biol. 1996; 16:7072–7083.
Article12. Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. Oncogene. 2003; 22:186–197.
Article13. Liu X, Zhao L, Derose YS, Lin YC, Bieniasz M, Eyob H, et al. Short-form ron promotes spontaneous breast cancer metastasis through interaction with phosphoinositide 3-kinase. Genes Cancer. 2011; 2:753–762.
Article14. Wang MH, Kurtz AL, Chen Y. Identification of a novel splicing product of the RON receptor tyrosine kinase in human colorectal carcinoma cells. Carcinogenesis. 2000; 21:1507–1512.
Article15. Lu Y, Yao HP, Wang MH. Multiple variants of the RON receptor tyrosine kinase: biochemical properties, tumorigenic activities, and potential drug targets. Cancer Lett. 2007; 257:157–164.
Article16. Zhang K, Zhou YQ, Yao HP, Wang MH. Alterations in a defined extracellular region of the RON receptor tyrosine kinase promote RON-mediated motile and invasive phenotypes in epithelial cells. Int J Oncol. 2010; 36:255–264.
Article17. Ghigna C, De Toledo M, Bonomi S, Valacca C, Gallo S, Apicella M, et al. Pro-metastatic splicing of Ron proto-oncogene mRNA can be reversed: therapeutic potential of bifunctional oligonucleotides and indole derivatives. RNA Biol. 2010; 7:495–503.
Article18. Yao HP, Zhou YQ, Zhang R, Wang MH. MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer. 2013; 13:466–481.
Article19. Faham N, Welm AL. RON signaling is a key mediator of tumor progression in many human cancers. Cold Spring Harb Symp Quant Biol. 2016; 81:177–188.
Article20. Shimamoto A, Kimura T, Matsumoto K, Nakamura T. Hepatocyte growth factor-like protein is identical to macrophage stimulating protein. FEBS Lett. 1993; 333:61–66.
Article21. Wang MH, Ronsin C, Gesnel MC, Coupey L, Skeel A, Leonard EJ, et al. Identification of the ron gene product as the receptor for the human macrophage stimulating protein. Science. 1994; 266:117–119.
Article22. Danilkovitch A, Miller M, Leonard EJ. Interaction of macrophage-stimulating protein with its receptor. Residues critical for beta chain binding and evidence for independent alpha chain binding. J Biol Chem. 1999; 274:29937–29943.23. Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994; 77:261–271.
Article24. Xiao ZQ, Chen YQ, Wang MH. Requirement of both tyrosine residues 1330 and 1337 in the C-terminal tail of the RON receptor tyrosine kinase for epithelial cell scattering and migration. Biochem Biophys Res Commun. 2000; 267:669–675.
Article25. Iwama A, Yamaguchi N, Suda T. STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking site conserved among the HGF receptor family. EMBO J. 1996; 15:5866–5875.
Article26. Santoro MM, Penengo L, Minetto M, Orecchia S, Cilli M, Gaudino G. Point mutations in the tyrosine kinase domain release the oncogenic and metastatic potential of the Ron receptor. Oncogene. 1998; 17:741–749.
Article27. McClaine RJ, Marshall AM, Wagh PK, Waltz SE. Ron receptor tyrosine kinase activation confers resistance to tamoxifen in breast cancer cell lines. Neoplasia. 2010; 12:650–658.
Article28. Logan-Collins J, Thomas RM, Yu P, Jaquish D, Mose E, French R, et al. Silencing of RON receptor signaling promotes apoptosis and gemcitabine sensitivity in pancreatic cancers. Cancer Res. 2010; 70:1130–1140.
Article29. Takeuchi K, Ito F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol Pharm Bull. 2011; 34:1774–1780.
Article30. O'Toole JM, Rabenau KE, Burns K, Lu D, Mangalampalli V, Balderes P, et al. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 2006; 66:9162–9170.31. Zhou D, Pan G, Zheng C, Zheng J, Yian L, Teng X. Expression of the RON receptor tyrosine kinase and its association with gastric carcinoma versus normal gastric tissues. BMC Cancer. 2008; 8:353.
Article32. Catenacci DV, Cervantes G, Yala S, Nelson EA, El-Hashani E, Kanteti R, et al. RON (MST1R) is a novel prognostic marker and therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol Ther. 2011; 12:9–46.
Article33. Song YA, Park YL, Kim KY, Myung E, Chung CY, Cho SB, et al. RON is associated with tumor progression via the inhibition of apoptosis and cell cycle arrest in human gastric cancer. Pathol Int. 2012; 62:127–136.
Article34. Lee J, Kang WK, Park JO, Park SH, Park YS, Lim HY, et al. Expression of activated signal transducer and activator of transcription 3 predicts poor clinical outcome in gastric adenocarcinoma. APMIS. 2009; 117:598–606.
Article35. Kim DY, Cha ST, Ahn DH, Kang HY, Kwon CI, Ko KH, et al. STAT3 expression in gastric cancer indicates a poor prognosis. J Gastroenterol Hepatol. 2009; 24:646–651.
Article36. Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol. 2010; 16:5380–5387.
Article37. Lee KE, Park JS, Khoi PN, Joo YE, Lee YH, Jung YD. Upregulation of recepteur d'origine nantais tyrosine kinase and cell invasiveness via early growth response-1 in gastric cancer cells. J Cell Biochem. 2012; 113:1217–1223.
Article38. Park JS, Park JH, Khoi PN, Joo YE, Jung YD. MSP-induced RON activation upregulates uPAR expression and cell invasiveness via MAPK, AP-1 and NF-κB signals in gastric cancer cells. Carcinogenesis. 2011; 32:175–181.
Article39. Park JS, Park JH, Lee S, Joo YE, Jung YD. Small interfering RNA targeting of Recepteur d'Origine Nantais induces apoptosis via modulation of nuclear factor-kappaB and Bcl-2 family in gastric cancer cells. Oncol Rep. 2010; 24:709–714.40. Kang CM, Babicky ML, Lowy AM. The RON receptor tyrosine kinase in pancreatic cancer pathogenesis and its potential implications for future targeted therapies. Pancreas. 2014; 43:183–189.
Article41. Kretschmann KL, Eyob H, Buys SS, Welm AL. The macrophage stimulating protein/Ron pathway as a potential therapeutic target to impede multiple mechanisms involved in breast cancer progression. Curr Drug Targets. 2010; 11:1157–1168.
Article42. Zhao S, Cao L, Freeman JW. Knockdown of RON receptor kinase delays but does not prevent tumor progression while enhancing HGF/MET signaling in pancreatic cancer cell lines. Oncogenesis. 2013; 2:e76.
Article43. Kataoka Y, Mukohara T, Tomioka H, Funakoshi Y, Kiyota N, Fujiwara Y, et al. Foretinib (GSK1363089), a multi-kinase inhibitor of MET and VEGFRs, inhibits growth of gastric cancer cell lines by blocking inter-receptor tyrosine kinase networks. Invest New Drugs. 2012; 30:1352–1360.
Article44. Shah MA, Wainberg ZA, Catenacci DV, Hochster HS, Ford J, Kunz P, et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS One. 2013; 8:e54014.
Article45. Awazu Y, Nakamura K, Mizutani A, Kakoi Y, Iwata H, Yamasaki S, et al. A novel inhibitor of c-Met and VEGF receptor tyrosine kinases with a broad spectrum of in vivo antitumor activities. Mol Cancer Ther. 2013; 12:913–924.
Article46. Toiyama Y, Yasuda H, Saigusa S, Matushita K, Fujikawa H, Tanaka K, et al. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int J Cancer. 2012; 130:2912–2921.
Article47. Yashiro M, Nishii T, Hasegawa T, Matsuzaki T, Morisaki T, Fukuoka T, et al. A c-Met inhibitor increases the chemosensitivity of cancer stem cells to the irinotecan in gastric carcinoma. Br J Cancer. 2013; 109:2619–2628.
Article48. Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003; 3:213–217.
Article49. Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002; 8:4 Suppl. S55–S61.
Article50. Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med (Berl). 2004; 82:488–499.
Article51. Germano S, Barberis D, Santoro MM, Penengo L, Citri A, Yarden Y, et al. Geldanamycins trigger a novel Ron degradative pathway, hampering oncogenic signaling. J Biol Chem. 2006; 281:21710–21719.
Article52. Moser C, Lang SA, Hackl C, Zhang H, Lundgren K, Hong V, et al. Oncogenic MST1R activity in pancreatic and gastric cancer represents a valid target of HSP90 inhibitors. Anticancer Res. 2012; 32:427–437.53. Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, et al. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001; 84:844–850.
Article54. Park JS, Khoi PN, Joo YE, Lee YH, Lang SA, Stoeltzing O, et al. EGCG inhibits recepteur d'origine nantais expression by suppressing Egr-1 in gastric cancer cells. Int J Oncol. 2013; 42:1120–1126.
Article55. Xia Y, Lian S, Khoi PN, Yoon HJ, Han JY, Chay KO, et al. Chrysin inhibits cell invasion by inhibition of Recepteur d'origine Nantais via suppressing early growth response-1 and NF-κB transcription factor activities in gastric cancer cells. Int J Oncol. 2015; 46:1835–1843.
Article56. Lian S, Park JS, Xia Y, Nguyen TT, Joo YE, Kim KK, et al. Micro-RNA-375 functions as a tumor-suppressor gene in gastric cancer by targeting recepteur d'Origine Nantais. Int J Mol Sci. 2016; 17:E1633.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Macrophage-Stimulating Protein Enhances Osteoblastic Differentiation via the Recepteur d'Origine Nantais Receptor and Extracellular Signal-Regulated Kinase Signaling Pathway
- Aspirin and Non-steroidal Anti-inflammatory Drugs in Prevention of Gastric Cancer
- Application of Long Non-coding RNAs in Gastric Cancer
- Helicobacter pylori-associated Chronic Atrophic Gastritis and Progression of Gastric Carcinogenesis
- Role of MRI in Gastric Cancer as a Diagnostic Tool