J Korean Med Sci.
2017 Nov;32(11):1807-1813. 10.3346/jkms.2017.32.11.1807.
Evaluation of the Efficacy and Safety of DA-9601 versus Its New Formulation, DA-5204, in Patients with Gastritis: Phase III, Randomized, Double-Blind, Non-Inferiority Study
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. dhljohn@yahoo.com
- 2Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Kangwon National University Hospital, Chuncheon, Korea.
- 4Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- 5Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea.
- 6Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 7Department of Internal Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 8Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 9Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 10Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea.
- 11Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea.
- 12Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea.
- 13Department of Internal Medicine, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 14Department of Internal Medicine, Inje University Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 15Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea.
- 16Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 17Department of Internal Medicine, Chonbuk University Medical School, Jeonju, Korea.
- 18Department of Internal Medicine, Presbyterian Medical Center, Jeonju, Korea.
- 19Department of Internal Medicine, Jeju National University Hospital, Jeju, Korea.
- 20Department of Internal Medicine, Hanyang University College Medicine, Seoul, Korea.
- 21Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2390307
- DOI: http://doi.org/10.3346/jkms.2017.32.11.1807
Abstract
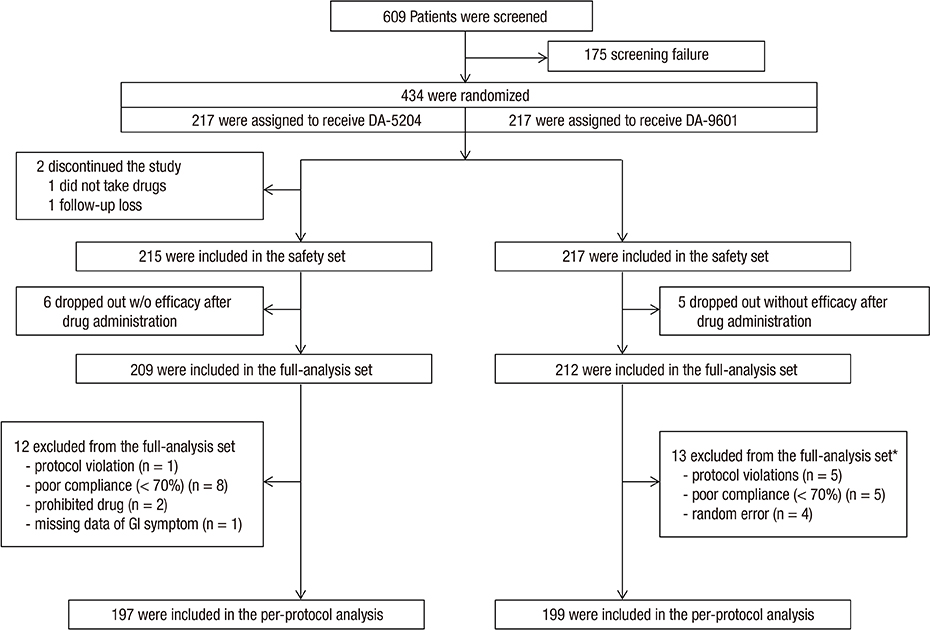
- This study compared the efficacy of DA-9601 (Dong-A ST Co., Seoul, Korea) and its new formulation, DA-5204 (Dong-A ST Co.), for treating erosive gastritis. This phase III, randomized, multicenter, double-blind, non-inferiority trial randomly assigned 434 patients with endoscopically proven gastric mucosal erosions into two groups: DA-9601 3 times daily or DA-5,204 twice daily for 2 weeks. The final analysis included 421 patients (DA-5204, 209; DA-9601, 212). The primary endpoint (rate of effective gastric erosion healing) and secondary endpoints (cure rate of endoscopic erosion and gastrointestinal [GI] symptom relief) were assessed using endoscopy after the treatment. Drug-related adverse events (AEs), including GI symptoms, were also compared. At week 2, gastric healing rates with DA-5204 and DA-9601 were 42.1% (88/209) and 42.5% (90/212), respectively. The difference between the groups was −0.4% (95% confidence interval, −9.8% to 9.1%), which was above the non-inferiority margin of −14%. The cure rate of gastric erosion in both groups was 37.3%. The improvement rates of GI symptoms with DA-5204 and DA-9601 were 40.4% and 40.8%, respectively. There were no statistically significant differences between the two groups in both secondary endpoints. AEs were reported in 18 (8.4%) patients in the DA-5204 group and 19 (8.8%) in the DA-9601 group. Rates of AE were not different between the two groups. No serious AE or adverse drug reaction (ADR) occurred. These results demonstrate the non-inferiority of DA-5204 compared to DA-9601. DA-5204 is as effective as DA-9601 in the treatment of erosive gastritis. Registered randomized clinical trial at ClinicalTrials.gov (NCT02282670)
Keyword
MeSH Terms
Figure
Reference
-
1. Seol SY, Kim MH, Ryu JS, Choi MG, Shin DW, Ahn BO. DA-9601 for erosive gastritis: results of a double-blind placebo-controlled phase III clinical trial. World J Gastroenterol. 2004; 10:2379–2382.2. Kim JS, Cha KH, Kang SY, Won D, Jang SW, Son M, Son MH, Choi HJ, Lee YW, Kang MJ. In vivo gastric residence and gastroprotective effect of floating gastroretentive tablet of DA-9601, an extract of Artemisia asiatica, in beagle dogs. Drug Des Devel Ther. 2016; 10:1917–1925.3. Jeong JJ, Choi MG, Choi H, Park JM, Oh JH, Jeon EJ, Lee BI, Lee IS, Kim SW, Choi SW, et al. Single blinded, randomized, active drug comparative, multi-center study to evaluate the therapeutic efficacy of gliptide (R) tab (sulglycotide 200 mg) in gastritis patients; phase IV study. Korean J Gastrointest Endosc. 2007; 35:125–132.4. Oh TY, Ahn BO, Ko JI, Ryu BK, Son MW, Kim SH, Kim WB, Lee EB. Studies on protective effect of DA-9601, an Artemisiae Herba extract, against ethanol-induced gastric mucosal damage and its mechanism. J Appl Pharmacol. 1997; 5:202–210.5. Oh TY, Ryu BK, Park JB, Lee SD, Kim WB, Yang J, Lee EB. Studies on antiulcer effects of DA-9601, an Artemisia herba extract, against experimental gastric ulcers and its mechanism. J Appl Pharmacol. 1996; 4:111–121.6. Oh TY, Ryu BK, Ko JI, Ahn BO, Kim SH, Kim WB, Lee EB, Jin JH, Ham KB. Protective effect of DA-9601, an extract of Artemisiae Herba, against naproxen-induced gastric damage in arthritic rats. Arch Pharm Res. 1997; 20:414–419.7. Choi SC, Choi EJ, Oh HM, Lee S, Lee JK, Lee MS, Shin YI, Choi SJ, Chae JR, Lee KM, et al. DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-alpha-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-kappaB pathways in human gastric epithelial cells. World J Gastroenterol. 2006; 12:4850–4858.8. Choi EJ, Oh HM, Na BR, Ramesh TP, Lee HJ, Choi CS, Choi SC, Oh TY, Choi SJ, Chae JR, et al. Eupatilin protects gastric epithelial cells from oxidative damage and down-regulates genes responsible for the cellular oxidative stress. Pharm Res. 2008; 25:1355–1364.9. Huh K, Kwon TH, Shin US, Kim WB, Ahn BO, Oh TY, Kim JA. Inhibitory effects of DA-9601 on ethanol-induced gastrohemorrhagic lesions and gastric xanthine oxidase activity in rats. J Ethnopharmacol. 2003; 88:269–273.10. Clarke G, Newton J, Short M. Gastrointestinal transit of pellets of differing size and density. Int J Pharm. 1993; 100:81–92.11. Akiyama Y, Nagahara N, Kashihara T, Hirai S, Toguchi H. In vitro and in vivo evaluation of mucoadhesive microspheres prepared for the gastrointestinal tract using polyglycerol esters of fatty acids and a poly (acrylic acid) derivative. Pharm Res. 1995; 12:397–405.12. J Urquhart F Theeuwes . Alza Corporation. Drug delivery system comprising a reservoir containing a plurality of tiny pills. United States patent. US 4434153 A. 1984. Feb. 28.13. Özdemir N, Ordu S, Özkan Y. Studies of floating dosage forms of furosemide: in vitro and in vivo evaluations of bilayer tablet formulations. Drug Dev Ind Pharm. 2000; 26:857–866.14. Toh MR, Teo V, Kwan YH, Raaj S, Tan SY, Tan JZ. Association between number of doses per day, number of medications and patient's non-compliance, and frequency of readmissions in a multi-ethnic Asian population. Prev Med Rep. 2014; 1:43–47.15. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001; 23:1296–1310.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention of NSAID-Associated Gastroduodenal Injury in Healthy Volunteers-A Randomized, Double-Blind, Multicenter Study Comparing DA-9601 with Misoprostol
- Augmenting Effect of DA-9601 on Ghrelin in an Acute Gastric Injury Model
- Correspondence to editorial on “Safety and efficacy of HK-660S in patients with primary sclerosing cholangitis: A randomized double-blind phase 2a trial”
- Suppressive Effects of Antioxidant DA-9601 on Hepatic Fibrosis in Rats
- Anti-inflammatory effects of DA-9601, an extract of Artemisia asiatica, on aceclofenac-induced acute enteritis