Korean J Physiol Pharmacol.
2021 Sep;25(5):439-448. 10.4196/kjpp.2021.25.5.439.
Anti-inflammatory effects of DA-9601, an extract of Artemisia asiatica, on aceclofenac-induced acute enteritis
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Dankook University, Cheonan 31116, Korea.
- 2Research Institute of Dong-A ST Co., Ltd., Yongin 17073, Korea.
- 3Department of Biochemistry, College of Medicine, Chung-Ang University, Seoul 06974, Korea.
- 4Department of Pathology, College of Medicine, Dankook University, Cheonan 31116, Korea.
- 5NeuroVis Inc., Cheonan 31035, Korea.
- KMID: 2519406
- DOI: http://doi.org/10.4196/kjpp.2021.25.5.439
Abstract
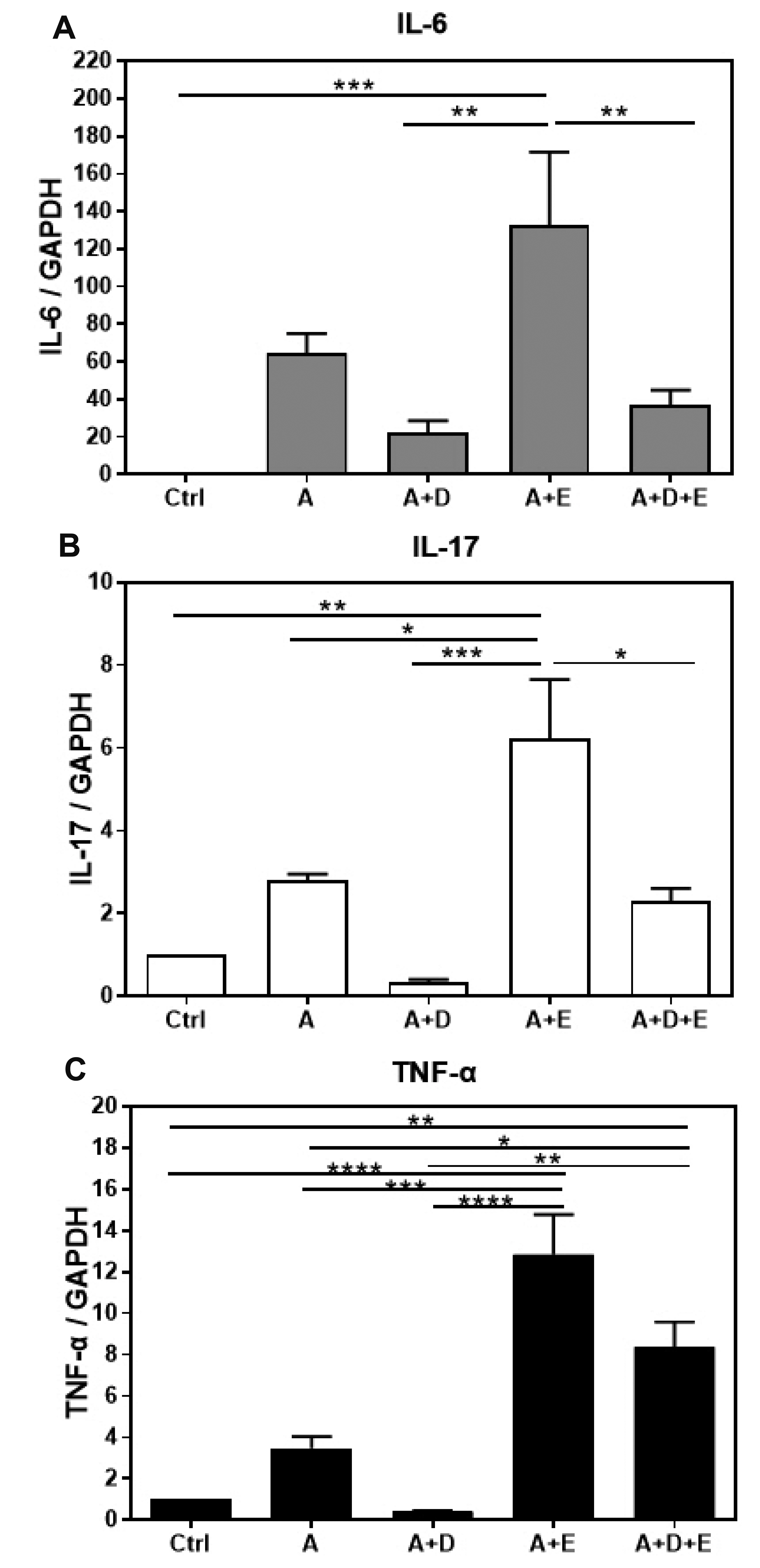
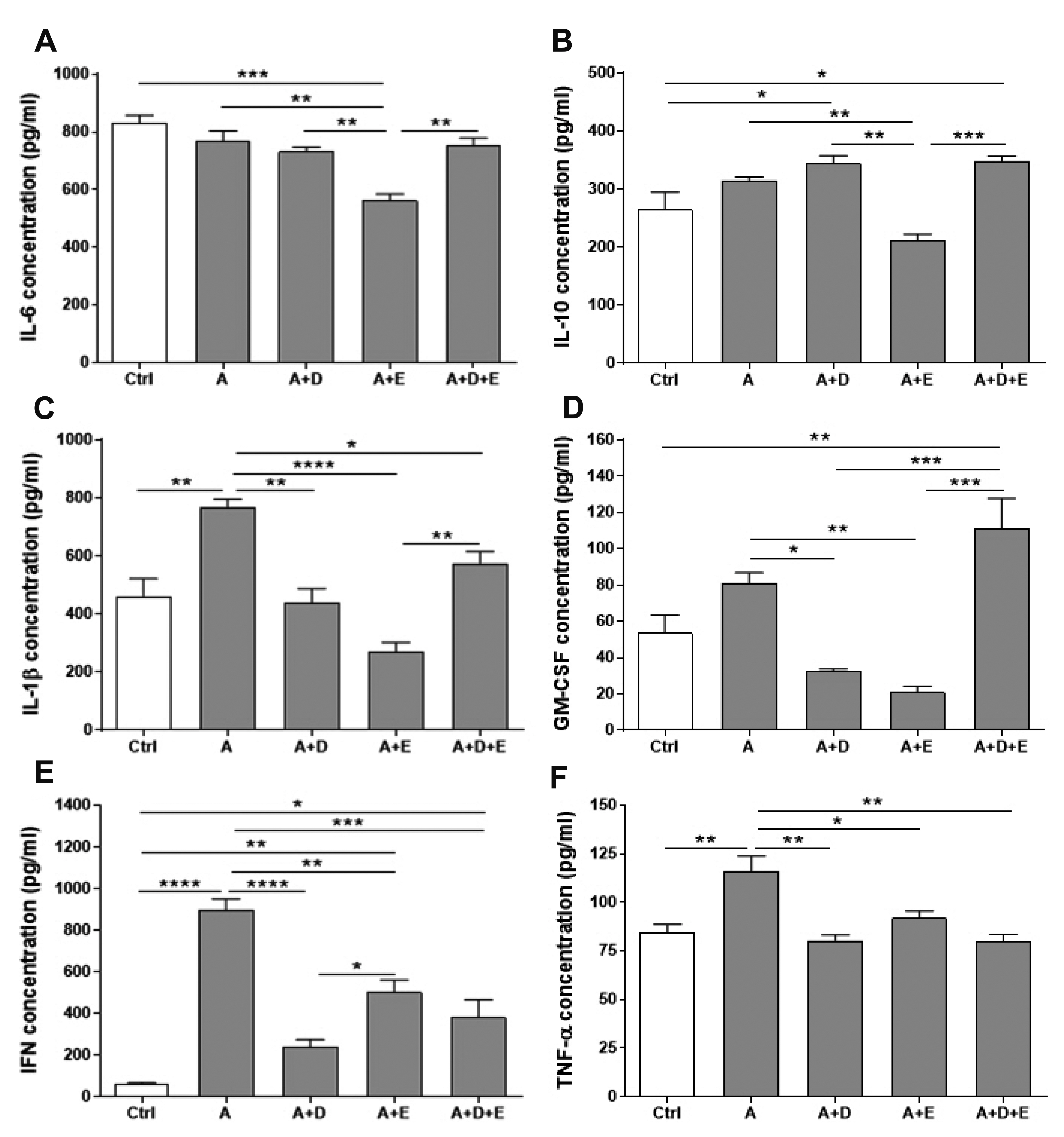
- DA-9601 is an extract obtained from Artemisia asiatica, which has been reported to have anti-inflammatory effects on gastrointestinal lesions; however, its possible anti-inflammatory effects on the small intestine have not been studied yet. Therefore, in this study, we investigated the protective effects of DA-9601 against the ACF-induced small intestinal inflammation. Inflammation of the small intestine was confirmed by histological studies and the changes in the CD4 + T cell fraction induced by the inflammation-related cytokines, and the inflammatory reactions were analyzed. Multifocal discrete small necrotic ulcers with intervening normal mucosa were frequently observed after treatment with ACF. The expression of IL-6, IL-17, and TNF-α genes was increased in the ACF group; however, it was found to have been significantly decreased in the DA-9601 treated group. In addition, DA-9601 significantly decreased the levels of proinflammatory mediators such as IL-1β, GMCSF, IFN-γ, and TNF-α; the anti-inflammatory cytokine IL-10, on the other hand, was observed to have increased. It is known that inflammatory mediators related to T cell imbalance and dysfunction continuously activate the inflammatory response, causing chronic tissue damage. The fractions of IFN-γ + Th1 cells, IL-4 + Th2 cells, IL-9 + Th9 cells, IL-17 + Th17 cells, and Foxp3 + Treg cells were significantly decreased upon DA-9601 treatment. These data suggest that the inflammatory response induced by ACF is reduced by DA-9601 via lowering of the expression of genes encoding the inflammatory cytokines and the concentration of inflammatory mediators. Furthermore, DA-9601 inhibited the acute inflammatory response mediated by T cells, resulting in an improvement in ACF-induced enteritis.
Keyword
Figure
Reference
-
1. M'Koma AE. 2013; Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol. 6:33–47. DOI: 10.4137/CGast.S12731. PMID: 24833941. PMCID: PMC4020403.2. Podolsky DK. 2002; Inflammatory bowel disease. N Engl J Med. 347:417–429. DOI: 10.1056/NEJMra020831. PMID: 12167685.
Article3. Jo-Watanabe A, Okuno T, Yokomizo T. 2019; The role of leukotrienes as potential therapeutic targets in allergic disorders. Int J Mol Sci. 20:3580. DOI: 10.3390/ijms20143580. PMID: 31336653. PMCID: PMC6679143.
Article4. Ananthakrishnan AN, Higuchi LM, Huang ES, Khalili H, Richter JM, Fuchs CS, Chan AT. 2012; Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med. 156:350–359. DOI: 10.7326/0003-4819-156-5-201203060-00007. PMID: 22393130. PMCID: PMC3369539.5. Laine L, Smith R, Min K, Chen C, Dubois RW. 2006; Systematic review: the lower gastrointestinal adverse effects of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 24:751–767. DOI: 10.1111/j.1365-2036.2006.03043.x. PMID: 16918879.
Article6. Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM, Verdu E, Ongini E. 2011; Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 141:1314–1322. 1322.e1-e5. DOI: 10.1053/j.gastro.2011.06.075. PMID: 21745447.
Article7. Satoh H, Amagase K, Takeuchi K. 2012; Exacerbation of nonsteroidal anti-inflammatory drug-induced small intestinal lesions by antisecretory drugs in rats: the role of intestinal motility. J Pharmacol Exp Ther. 343:270–277. DOI: 10.1124/jpet.112.197475. PMID: 22854201.
Article8. Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Alonso-Abreu I, Perez-Aisa MA, Perez-Gisbert J, Bujanda L, Castro M, Muñoz M, Rodrigo L, Calvet X, Del-Pino D, Garcia S. 2009; Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 104:1633–1641. DOI: 10.1038/ajg.2009.164. PMID: 19574968.
Article9. Legrand E. 2004; Aceclofenac in the management of inflammatory pain. Expert Opin Pharmacother. 5:1347–1357. DOI: 10.1517/14656566.5.6.1347. PMID: 15163279.
Article10. Lee OY, Kang DH, Lee DH, Chung IK, Jang JY, Kim JI, Cho JW, Rew JS, Lee KM, Kim KO, Choi MG, Lee SW, Lee ST, Kim TO, Shin YW, Seol SY. 2014; A comparative study of DA-9601 and misoprostol for prevention of NSAID-associated gastroduodenal injury in patients undergoing chronic NSAID treatment. Arch Pharm Res. 37:1308–1316. DOI: 10.1007/s12272-014-0408-3. PMID: 24871787. PMCID: PMC4176566.
Article11. Huh K, Kwon TH, Shin US, Kim WB, Ahn BO, Oh TY, Kim JA. 2003; Inhibitory effects of DA-9601 on ethanol-induced gastrohemorrhagic lesions and gastric xanthine oxidase activity in rats. J Ethnopharmacol. 88:269–273. DOI: 10.1016/S0378-8741(03)00235-6. PMID: 12963154.
Article12. Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Lee KM, Hahm KB. 2001; Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 49:364–371. DOI: 10.1136/gut.49.3.364. PMID: 11511558. PMCID: PMC1728432.
Article13. Boynton CS, Dick CF, Mayor GH. 1988; NSAIDs: an overview. J Clin Pharmacol. 28:512–517. DOI: 10.1002/j.1552-4604.1988.tb03170.x. PMID: 3047176.
Article14. Scheiman JM. 2013; The use of proton pump inhibitors in treating and preventing NSAID-induced mucosal damage. Arthritis Res Ther. 15(Suppl 3):S5. DOI: 10.1186/ar4177. PMID: 24267413. PMCID: PMC3891010.
Article15. Tai FWD, McAlindon ME. 2018; NSAIDs and the small bowel. Curr Opin Gastroenterol. 34:175–182. DOI: 10.1097/MOG.0000000000000427. PMID: 29438118.
Article16. Park SC, Chun HJ, Kang CD, Sul D. 2011; Prevention and management of non-steroidal anti-inflammatory drugs-induced small intestinal injury. World J Gastroenterol. 17:4647–4653. DOI: 10.3748/wjg.v17.i42.4647. PMID: 22180706. PMCID: PMC3237301.
Article17. Oh TY, Ryu BK, Ko JI, Ahn BO, Kim SH, Kim WB, Lee EB, Jin JH, Hahm KB. 1997; Protective effect of DA-9601, an extract of Artemisiae Herba, against naproxen-induced gastric damage in arthritic rats. Arch Pharm Res. 20:414–419. DOI: 10.1007/BF02973932. PMID: 18982482.18. Kim HS, Kundu JK, Lee JS, Oh TY, Na HK, Surh YJ. 2008; Chemopreventive effects of the standardized extract (DA-9601) of Artemisia asiatica on azoxymethane-initiated and dextran sulfate sodium-promoted mouse colon carcinogenesis. Nutr Cancer. 60 Suppl 1:90–97. DOI: 10.1080/01635580802404170. PMID: 19003585.19. Laine L. 1996; Nonsteroidal anti-inflammatory drug gastropathy. Gastrointest Endosc Clin N Am. 6:489–504. DOI: 10.1016/S1052-5157(18)30351-9. PMID: 8803564.
Article20. Blackler RW, Gemici B, Manko A, Wallace JL. 2014; NSAID-gastroenteropathy: new aspects of pathogenesis and prevention. Curr Opin Pharmacol. 19:11–16. DOI: 10.1016/j.coph.2014.05.008. PMID: 24929967.
Article21. Gwee KA, Goh V, Lima G, Setia S. 2018; Coprescribing proton-pump inhibitors with nonsteroidal anti-inflammatory drugs: risks versus benefits. J Pain Res. 11:361–374. DOI: 10.2147/JPR.S156938. PMID: 29491719. PMCID: PMC5817415.
Article22. Liu T, Zhang L, Joo D, Sun SC. 2017; NF-κB signaling in inflammation. Signal Transduct Target Ther. 2:17023. DOI: 10.1038/sigtrans.2017.23. PMID: 29158945. PMCID: PMC5661633.
Article23. Műzes G, Molnár B, Tulassay Z, Sipos F. 2012; Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol. 18:5848–5861. DOI: 10.3748/wjg.v18.i41.5848. PMID: 23139600. PMCID: PMC3491591.
Article24. Strober W, Fuss IJ. 2011; Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 140:1756–1767. DOI: 10.1053/j.gastro.2011.02.016. PMID: 21530742. PMCID: PMC3773507.
Article25. Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T. 2017; The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediators Inflamm. 2017:3908061. DOI: 10.1155/2017/3908061. PMID: 28316374. PMCID: PMC5337858.
Article26. Chamoun-Emanuelli AM, Bryan LK, Cohen ND, Tetrault TL, Szule JA, Barhoumi R, Whitfield-Cargile CM. 2019; NSAIDs disrupt intestinal homeostasis by suppressing macroautophagy in intestinal epithelial cells. Sci Rep. 9:14534. DOI: 10.1038/s41598-019-51067-2. PMID: 31601922. PMCID: PMC6787209.
Article27. Song EM, Jung SA, Lee JS, Kim SE, Shim KN, Jung HK, Yoo K, Park HY. 2013; Benzoxazole derivative B-98 ameliorates dextran sulfate sodium-induced acute murine colitis and the change of T cell profiles in acute murine colitis model. Korean J Gastroenterol. 62:33–41. DOI: 10.4166/kjg.2013.62.1.33. PMID: 23954958.
Article28. Yamada A, Arakaki R, Saito M, Tsunematsu T, Kudo Y, Ishimaru N. 2016; Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 22:2195–2205. DOI: 10.3748/wjg.v22.i7.2195. PMID: 26900284. PMCID: PMC4734996.
Article29. Cătană CS, Berindan Neagoe I, Cozma V, Magdaş C, Tăbăran F, Dumitraşcu DL. 2015; Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 21:5823–5830. DOI: 10.3748/wjg.v21.i19.5823. PMID: 26019446. PMCID: PMC4438016.
Article30. Lee SH, Kwon JE, Cho ML. 2018; Immunological pathogenesis of inflammatory bowel disease. Intest Res. 16:26–42. DOI: 10.5217/ir.2018.16.1.26. PMID: 29422795. PMCID: PMC5797268.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Augmenting Effect of DA-9601 on Ghrelin in an Acute Gastric Injury Model
- Suppressive Effects of Antioxidant DA-9601 on Hepatic Fibrosis in Rats
- Eupatilin Ameliorates Collagen Induced Arthritis
- Prevention of NSAID-Associated Gastroduodenal Injury in Healthy Volunteers-A Randomized, Double-Blind, Multicenter Study Comparing DA-9601 with Misoprostol
- Anti-inflammatory effects of ethanolic extract of Annona muricata