Clin Endosc.
2017 May;50(3):261-269. 10.5946/ce.2016.056.
Characteristics of Missed Simultaneous Gastric Lesions Based on Double-Check Analysis of the Endoscopic Image
- Affiliations
-
- 1Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2388823
- DOI: http://doi.org/10.5946/ce.2016.056
Abstract
- BACKGROUND/AIMS
The detection of multifocal lesions is important for the successful management of gastric neoplasms. We investigated the characteristics of missed simultaneous lesions and the reason for the missed diagnoses.
METHODS
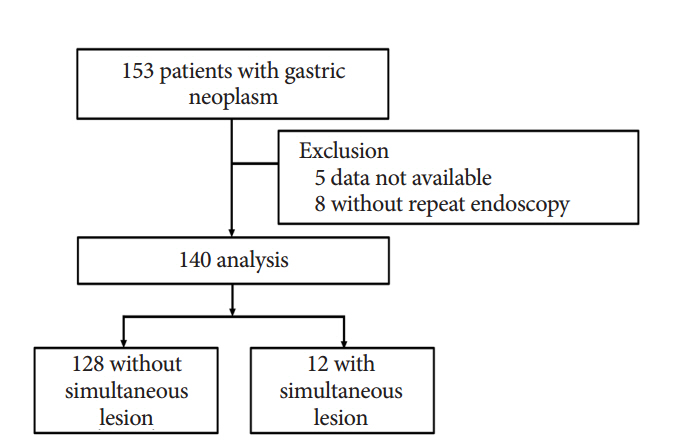
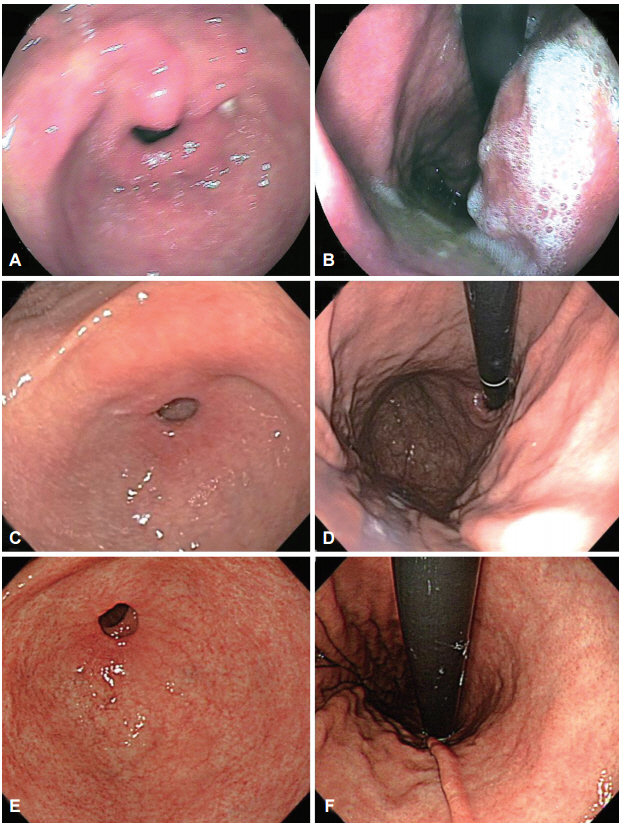
A total of 140 patients who underwent repeat endoscopy before endoscopic resection between June 2013 and June 2014 were retrospectively reviewed. We classified simultaneous lesions into three groups based on a review of earlier images: group 1, no images of the location of simultaneous lesions were taken; group 2, no corresponding lesion was evident in the previous images; and group 3, simultaneous lesions were visible in the earlier images but a biopsy was not performed.
RESULTS
Simultaneous lesions were found in 12 patients (8.6%) with 13 lesions, comprising 10 dysplasia (76.9%) and three adenocarcinoma (23.1%). Regarding the reasons for missed diagnoses, seven lesions (53.8%) were classified as group 3, five (38.5%) as group 1, and the remaining lesion (7.7%) as group 2. There were no significant differences in the characteristics of the patients with and without simultaneous lesions.
CONCLUSIONS
Lesions disregarded or unnoticed during endoscopic examination were the main reason for missed diagnosis of simultaneous lesions. Endoscopists should consider the possibility of simultaneous lesions and attempt to meticulously evaluate the entire gastric mucosa.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Common Locations of Gastric Cancer: Review of Research from the Endoscopic Submucosal Dissection Era
Su Jin Kim, Cheol Woong Choi
J Korean Med Sci. 2019;34(35):. doi: 10.3346/jkms.2019.34.e231.Characteristics of Synchronous and Metachronous Multiple Gastric Tumors after Endoscopic Submucosal Dissection of Early Gastric Neoplasm
Hyun Jik Lee, Yoo Jin Lee, Ju Yup Lee, Eun Soo Kim, Woo Jin Chung, Byoung Kuk Jang, Kyung Sik Park, Jae Seok Hwang, Kwang Bum Cho
Clin Endosc. 2018;51(3):266-273. doi: 10.5946/ce.2017.109.Characteristics of Missed Synchronous Gastric Epithelial Neoplasms
Bong Eun Lee
Clin Endosc. 2017;50(3):211-212. doi: 10.5946/ce.2017.058.
Reference
-
1. Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011; 74:485–493.
Article2. Choi MK, Kim GH, Park DY, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc. 2013; 27:4250–4258.
Article3. Gong EJ, Ahn JY, Jung HY, et al. Risk factors and clinical outcomes of gastric cancer identified by screening endoscopy: a case-control study. J Gastroenterol Hepatol. 2014; 29:301–309.
Article4. Voutilainen ME, Juhola MT. Evaluation of the diagnostic accuracy of gastroscopy to detect gastric tumours: clinicopathological features and prognosis of patients with gastric cancer missed on endoscopy. Eur J Gastroenterol Hepatol. 2005; 17:1345–1349.
Article5. Lee HL, Eun CS, Lee OY, et al. When do we miss synchronous gastric neoplasms with endoscopy? Gastrointest Endosc. 2010; 71:1159–1165.
Article6. Kim HH, Cho EJ, Noh E, et al. Missed synchronous gastric neoplasm with endoscopic submucosal dissection for gastric neoplasm: experience in our hospital. Dig Endosc. 2013; 25:32–38.
Article7. Kim HH, Kim JH, Kim GH, Choi MG, Jee SR, Song GA. Causes of missed synchronous gastric epithelial neoplasms with endoscopic submucosal dissection: a multicenter study. Scand J Gastroenterol. 2013; 48:1339–1346.
Article8. Yoo JH, Shin SJ, Lee KM, et al. How can we predict the presence of missed synchronous lesions after endoscopic submucosal dissection for early gastric cancers or gastric adenomas? J Clin Gastroenterol. 2013; 47:e17–e22.
Article9. Hosokawa O, Watanabe K, Hatorri M, Douden K, Hayashi H, Kaizaki Y. Detection of gastric cancer by repeat endoscopy within a short time after negative examination. Endoscopy. 2001; 33:301–305.
Article10. Yalamarthi S, Witherspoon P, McCole D, Auld CD. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy. 2004; 36:874–879.
Article11. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.12. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969; 1:87–97.
Article13. Sung IK, Kim YC, Yun JW, et al. Characteristics of advanced gastric cancer undetected on gastroscopy. Korean J Gastroenterol. 2011; 57:288–293.
Article14. Ren W, Yu J, Zhang ZM, Song YK, Li YH, Wang L. Missed diagnosis of early gastric cancer or high-grade intraepithelial neoplasia. World J Gastroenterol. 2013; 19:2092–2096.
Article15. Kato M, Kaise M, Yonezawa J, et al. Magnifying endoscopy with narrow-band imaging achieves superior accuracy in the differential diagnosis of superficial gastric lesions identified with white-light endoscopy: a prospective study. Gastrointest Endosc. 2010; 72:523–529.
Article16. Zhang Q, Wang F, Chen ZY, et al. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: a meta-analysis. Gastric Cancer. 2016; 19:543–552.
Article17. Zhao Z, Yin Z, Wang S, et al. Meta-analysis: the diagnostic efficacy of chromoendoscopy for early gastric cancer and premalignant gastric lesions. J Gastroenterol Hepatol. 2016; Feb. 9. [Epub]. https://doi.org/10.1111/jgh.13313.
Article18. Rizk MK, Sawhney MS, Cohen J, et al. Quality indicators common to all GI endoscopic procedures. Gastrointest Endosc. 2015; 81:3–16.
Article19. Yang MJ, Shin SJ, Lee KS, et al. Non-neoplastic pathology results after endoscopic submucosal dissection for gastric epithelial dysplasia or early gastric cancer. Endoscopy. 2015; 47:598–604.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosing Gastric Mesenchymal Tumors by Digital Endoscopic Ultrasonography Image Analysis
- Review of Simultaneous Double Stenting Using Endoscopic Ultrasound-Guided Biliary Drainage Techniques in Combined Gastric Outlet and Biliary Obstructions
- Common Locations of Gastric Cancer: Review of Research from the Endoscopic Submucosal Dissection Era
- Approach for the Management of Indefinite-for-neoplasia Lesions Detected on Gastric Mucosal Biopsy
- A study on double contrast study in early gastric cancer