Korean J Physiol Pharmacol.
2016 Mar;20(2):177-183. 10.4196/kjpp.2016.20.2.177.
Identification of Lys49-PLA2 from crude venom of Crotalus atrox as a human neutrophil-calcium modulating protein
- Affiliations
-
- 1Department of Pharmacology, Institute of Natural Medicine, College of Medicine, Hallym University, Chuncheon 24252, Korea. limss@hallym.ac.kr dksong@hallym.ac.kr
- 2ProteomeTech Inc., Seoul 03722, Korea.
- 3Department of Food Science and Nutrition, Hallym University, Chuncheon 24252, Korea. limss@hallym.ac.kr dksong@hallym.ac.kr
- KMID: 2388674
- DOI: http://doi.org/10.4196/kjpp.2016.20.2.177
Abstract
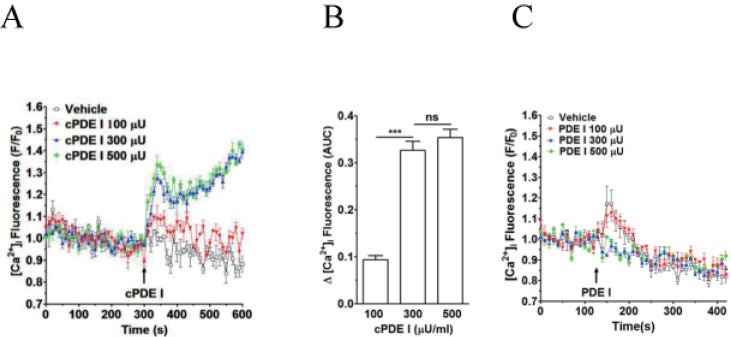
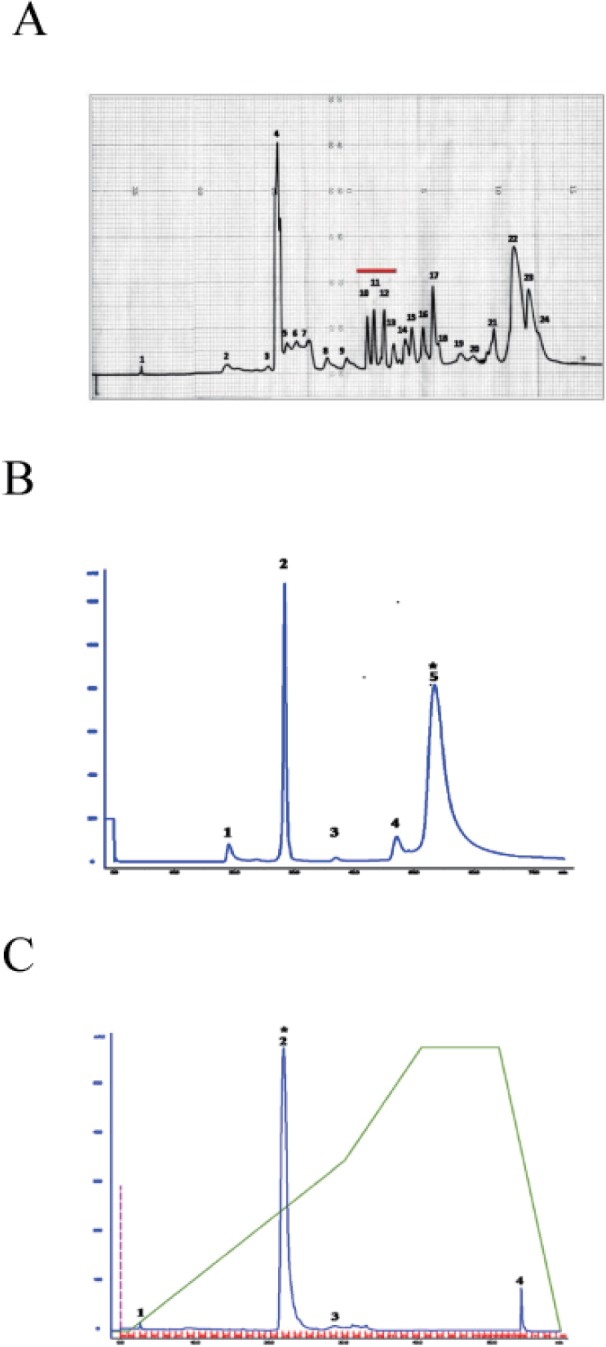
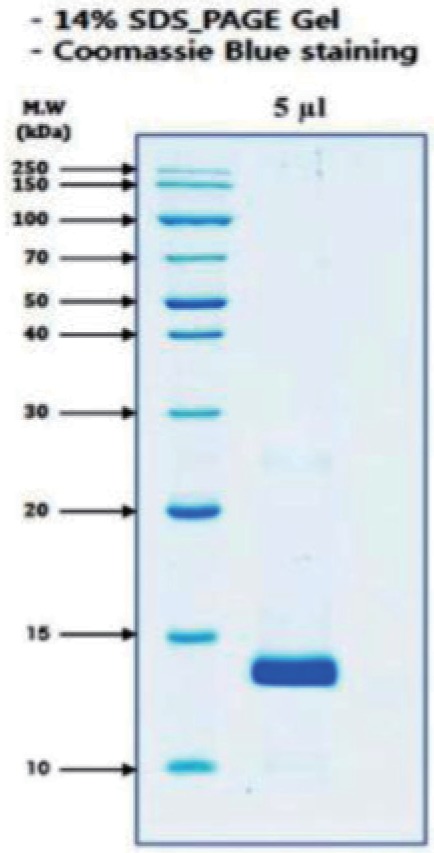
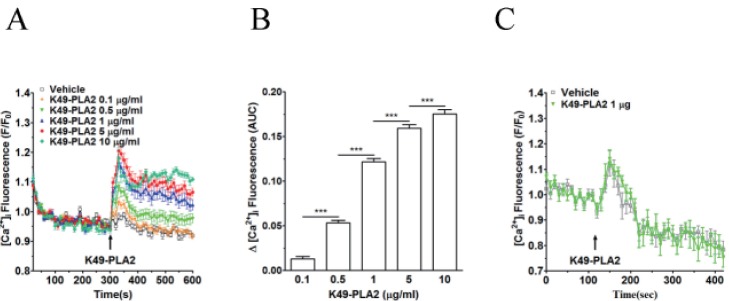
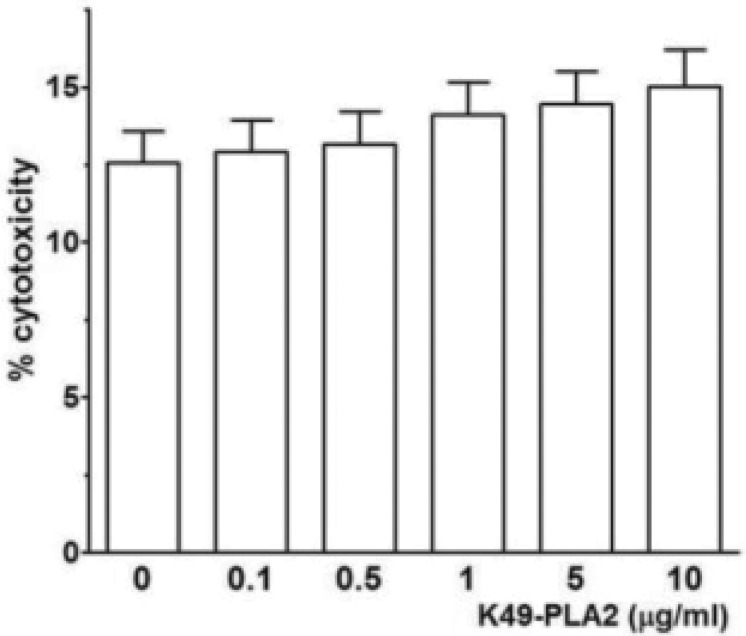
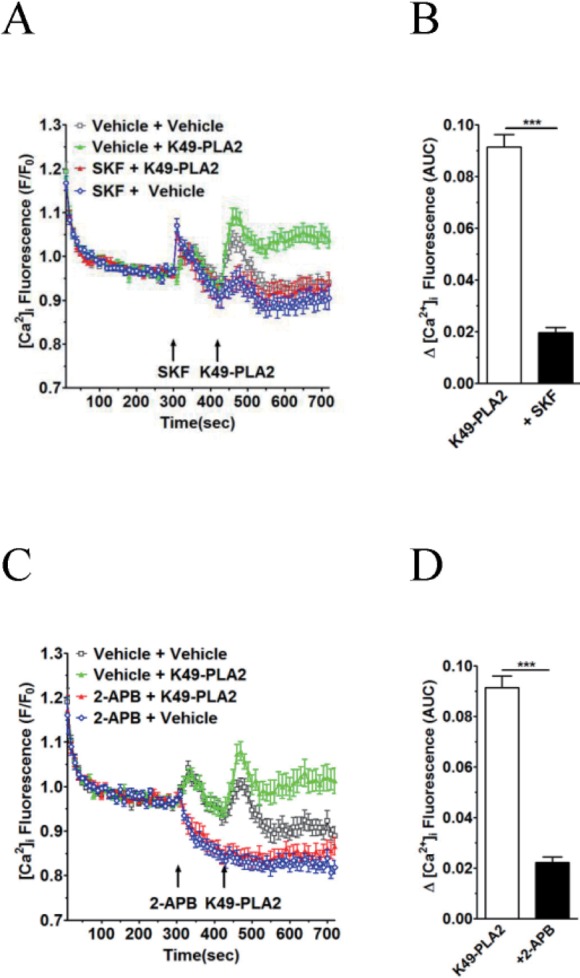
- We fortuitously observed a human neutrophil intracellular free-calcium concentration ([Ca2+]i) increasing activity in the commercially available phosphodiesterase I (PDE I), which is actually dried crude venom of Crotalus atrox. As this activity was not observed with another commercially available pure PDE I, we tried to find out the causative molecule(s) present in 'crude' PDE, and identified Lys49-phospholipase A2 (Lys49-PLA2 or K49-PLA2), a catalytically inactive protein which belongs to the phospholipase A2 family, by activity-driven three HPLC (reverse phase, size exclusion, reverse phase) steps followed by SDS-PAGE and LC-MS/MS. K49-PLA2 induced Ca2+ infl ux in human neutrophils without any cytotoxic eff ect. Two calcium channel inhibitors, 2-aminoetoxydiphenyl borate (2-APB) (30 microM) and SKF-96365 (20 microM) signifi cantly inhibited K49-PLA2-induced [Ca2+]i increase. These results suggest that K49-PLA2 modulates [Ca2+]i in human neutrophils via 2-APB- and SKF-96365-sensitive calcium channels without causing membrane disruption.
Keyword
MeSH Terms
Figure
Reference
-
1. Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005; 23:197–223. PMID: 15771570.
Article2. Berridge MJ, Bootman MD, Lipp P. Calcium-a life and death signal. Nature. 1998; 395:645–648. PMID: 9790183.3. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000; 1:11–21. PMID: 11413485.
Article4. Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997; 77:901–930. PMID: 9354808.
Article5. Salmon MD, Ahluwalia J. Pharmacology of receptor operated calcium entry in human neutrophils. Int Immunopharmacol. 2011; 11:145–148. PMID: 21081191.
Article6. Calvete JJ, Fasoli E, Sanz L, Boschetti E, Righetti PG. Exploring the venom proteome of the western diamondback rattlesnake, Crotalus atrox, via snake venomics and combinatorial peptide ligand library approaches. J Proteome Res. 2009; 8:3055–3067. PMID: 19371136.
Article7. Bahk YY, Kim SA, Kim JS, Euh HJ, Bai GH, Cho SN, Kim YS. Antigens secreted from Mycobacterium tuberculosis: identification by proteomics approach and test for diagnostic marker. Proteomics. 2004; 4:3299–3307. PMID: 15378731.8. Ménez A, Stöcklin R, Mebs D. 'Venomics' or : The venomous systems genome project. Toxicon. 2006; 47:255–259. PMID: 16460774.9. Lomonte B, Tsai WC, Ureña-Diaz JM, Sanz L, Mora-Obando D, Sánchez EE, Fry BG, Gutiérrez JM, Gibbs HL, Sovic MG, Calvete JJ. Venomics of New World pit vipers: genus-wide comparisons of venom proteomes across Agkistrodon. J Proteomics. 2014; 96:103–116. PMID: 24211403.
Article10. Lomonte B, Angulo Y, Sasa M, Gutiérrez JM. The phospholipase A2 homologues of snake venoms: biological activities and their possible adaptive roles. Protein Pept Lett. 2009; 16:860–876. PMID: 19689412.
Article11. Mora R, Maldonado A, Valverde B, Gutiérrez JM. Calcium plays a key role in the effects induced by a snake venom Lys49 phospholipase A2 homologue on a lymphoblastoid cell line. Toxicon. 2006; 47:75–86. PMID: 16303159.
Article12. Ward RJ, Chioato L, de Oliveira AH, Ruller R, Sá JM. Activesite mutagenesis of a Lys49-phospholipase A2: biological and membrane-disrupting activities in the absence of catalysis. Biochem J. 2002; 362:89–96. PMID: 11829743.
Article13. Petan T, Krizaj I, Pungercar J. Restoration of enzymatic activity in a Ser-49 phospholipase A2 homologue decreases its Ca2+-independent membrane-damaging activity and increases its toxicity. Biochemistry. 2007; 46:12795–12809. PMID: 17927217.14. Tsai IH, Chen YH, Wang YM, Tu MC, Tu AT. Purification, sequencing, and phylogenetic analyses of novel Lys-49 phospholipases A(2) from the venoms of rattlesnakes and other pit vipers. Arch Biochem Biophys. 2001; 394:236–244. PMID: 11594738.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Toxicity of crude and detoxified Tityus serrulatus venom in anti-venom-producing sheep
- Pretreatment of Diltiazem Ameliorates Endotoxin-Induced Acute Lung Injury by Suppression of Neutrophilic Oxidative Stress
- Effect of the inhibition of PLA2 and PAF on the neutrophilic respiratory burst and apoptosis
- Expression of Neutrophil-activating Chemokines in Human Neutrophils by Helicobacter pylori Water Extract
- Mediation of intracellular Ca2+ in the phospholipase A2-induced cell proliferation in human neuroblastoma cells