J Vet Sci.
2016 Dec;17(4):467-477. 10.4142/jvs.2016.17.4.467.
Toxicity of crude and detoxified Tityus serrulatus venom in anti-venom-producing sheep
- Affiliations
-
- 1Department of Veterinary Medicine, College of Veterinary Medicine, Federal University of Minas Gerais (UFMG), Belo Horizonte, MG 31275-013, Brazil. mariliamm@ufmg.br
- 2Department of Biochemistry and Immunology, Institute of Biological Sciences, Federal University of Minas Gerais (UFMG), Belo Horizonte, MG 31270-901, Brazil.
- 3Department of Veterinary Medicine, State University of Santa Cruz (UESC), Ilhéus, BA 45662-900, Brazil.
- KMID: 2412602
- DOI: http://doi.org/10.4142/jvs.2016.17.4.467
Abstract
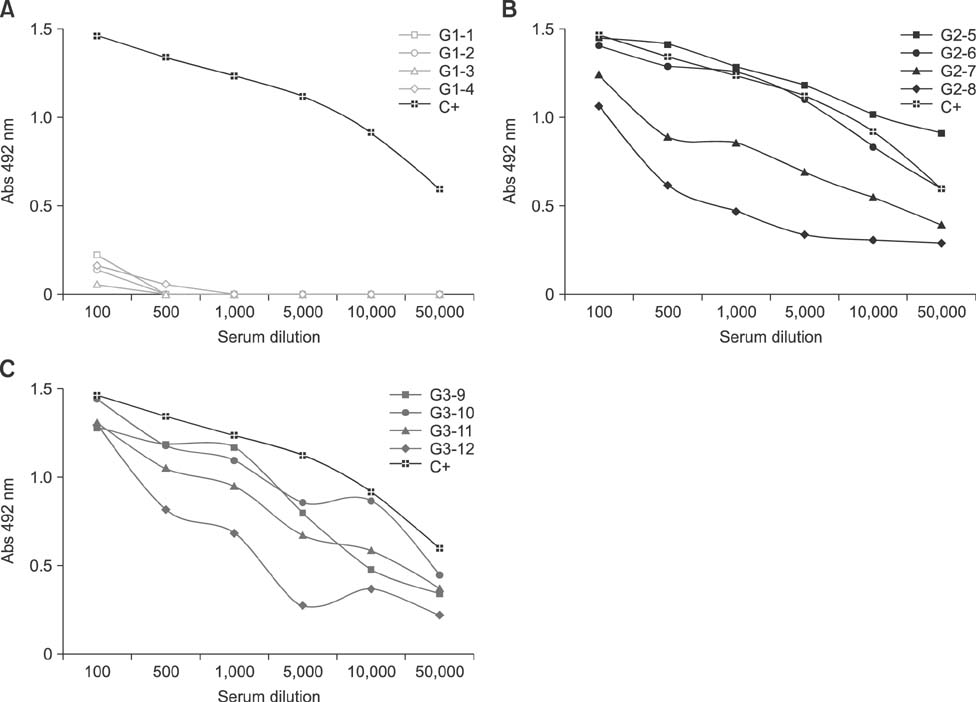
- Specific anti-venom used to treat scorpion envenomation is usually obtained from horses after hyperimmunization with crude scorpion venom. However, immunized animals often become ill because of the toxic effects of the immunogens used. This study was conducted to evaluate the toxic and immunogenic activities of crude and detoxified Tityus serrulatus (Ts) venom in sheep during the production of anti-scorpionic anti-venom. Sheep were categorized into three groups: G1, control, immunized with buffer only; G2, immunized with crude Ts venom; and G3, immunized with glutaraldehyde-detoxified Ts venom. All animals were subjected to clinical exams and supplementary tests. G2 sheep showed mild clinical changes, but the other groups tolerated the immunization program well. Specific antibodies generated in animals immunized with either Ts crude venom or glutaraldehyde-detoxified Ts venom recognized the crude Ts venom in both assays. To evaluate the lethality neutralization potential of the produced sera, individual serum samples were pre-incubated with Ts crude venom, then subcutaneously injected into mice. Efficient immune protection of 56.3% and 43.8% against Ts crude venom was observed in G2 and G3, respectively. Overall, the results of this study support the use of sheep and glutaraldehyde-detoxified Ts venom for alternative production of specific anti-venom.
MeSH Terms
Figure
Reference
-
1. Avrameas S, Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorvents. Immunochemistry. 1969; 6:53–66.
Article2. Becerril B, Marangoni S, Possani LD. Toxins and genes isolated from scorpions of the genus Tityus. Toxicon. 1997; 35:821–835.
Article3. Bouaziz M, Bahloul M, Kallel H, Samet M, Ksibi H, Dammak H, Ahmed MNB, Chtara K, Chelly H, Hamida CB, Rekik N. Epidemiological, clinical characteristics and outcome of severe scorpion envenomation in South Tunisia: multivariate analysis of 951 cases. Toxicon. 2008; 52:918–926.
Article4. Chávez-Olórtegui C, Kalapothakis E, Ferreira AMBM, Ferreira AP, Diniz CR. Neutralizing capacity of antibodies elicited by a non-toxic protein purified from the venom of the scorpion Tityus serrulatus. Toxicon. 1997; 35:213–221.
Article5. Chippaux JP, Goyffon M. Venoms, antivenoms, and immunotherapy. Toxicon. 1998; 36:823–846.
Article6. Cupo P, Hering SE. Cardiac troponin I release after severe scorpion envenoming by Tityus serrulatus. Toxicon. 2002; 40:823–830.
Article7. Cusinato DAC, Souza AM, Vasconcelos F, Guimarães LFL, Leite FP, Gregório ZMO, Giglio JR, Arantes EC. Assessment of biochemical and hematological parameters in rats injected with Tityus serrulatus scorpion venom. Toxicon. 2010; 56:1477–1486.
Article8. de Sousa Alves R, do Nascimento NRF, Barbosa PSF, Kerntopf MR, Lessa LMA, de Sousa CM, Martins RD, Sousa DF, de Queiroz MGR, Toyama MH, Fonteles MC, Martins AMC, Monteiro HSA. Renal effects and vascular reactivity induced by Tityus serrulatus venom. Toxicon. 2005; 46:271–276.
Article9. Fan HW, França FOS. Serotherapy. In : Schvartsman S, editor. Poisonous Plants and Venomous Animals. São Paulo: Sarvier;1992. p. 176–181.10. Flecknell P. Replacement, reduction and refinement. ALTEX. 2002; 19:73–78.11. Gazarian KG, Gazarian T, Hernández R, Possani LD. Immunology of scorpion toxins and perspectives for generation of anti-venom vaccines. Vaccine. 2005; 23:3357–3368.
Article12. Guidolin R, Da Silva WD, Higashi HG, Caricati CP, Lima MLSR, Morais JF, Pinto JR, Marcelino JR. Hyperimmunization of horses producing anti-venom serum with botrypic and crotalic venoms. Mem Inst Butantan. 1989; 51:85–90.13. Guimarães PT, Pinto MCL, Labarrère CR, Verçosa D Jr, Melo MM. Biochemical and anatomo-histopathogical alterations caused by Tityus fasciolatus scorpion venom in mice. Rev Bras Cien Vet. 2009; 16:51–57.14. Hering SE, Azevedo-Marques MM, Cupo P. Scorpionism. In : Schvartsman S, editor. Poisonous Plants and Venomous Animals. São Paulo: Sarvier;1992. p. 216–227.15. Ismail M, Abd-Elsalam MA. Are the toxicological effects of scorpion envenomation related to tissue venom concentration? Toxicon. 1988; 26:233–256.
Article16. Jackson PGG, Cockcroft PD. Clinical Examination of Farm Animals. Ames: Blackwell Publishing Company;2002.17. Kaneko JJ, Harvey JW, Bruss ML. Clinical Biochemistry of Domestic Animals. San Diego: Academic Press;2008.18. Lago EP, Melo MM, Araújo RB, Nascimento EF, Silva EF, Melo MB. Electrocardiographical and echocardiographical profiles in experimental Mascagnia rigida Griseb. (Malpighiaceae) toxicosis in sheep. Arq Bras Med Vet Zootec. 2009; 61:853–862.19. Landon J, Smith DS. Merits of sheep antisera for antivenom manufacture. J Toxicol Toxin Rev. 2003; 22:15–22.
Article20. Machado de Avila RA, Alvarenga LM, Tavares CAP, Molina F, Granier C, Chávez-Olórtegui C. Molecular characterization of protective antibodies raised in mice by Tityus serrulatus scorpion venom toxins conjugated to bovine serum albumin. Toxicon. 2004; 44:233–241.
Article21. Marcussi S, Arantes EC, Soares AM. Scorpions: Biology, Poisoning and Action Mechanism of Their Toxins. São Paulo: FUNPEC Editora;2011. p. 140.22. Maria WS, Velarde DT, Alvarenga LM, Nguyen C, Villard S, Granier C, Chávez-Olórtegui C. Localization of epitopes in the toxins of Tityus serrulatus scorpions and neutralizing potential of therapeutic antivenoms. Toxicon. 2005; 46:210–217.
Article23. Novaes G, Queiroz AC, Queiroz AP, Santos RR, Flores DG, Henkes G, Fernandes BJD. Histopathologic study in rats' pancrease after injection of venom from the scorpion Tityus serrulatus. Rev Cien Med Biol. 2002; 1:1–6.24. Pessini AC, Santos DR, Arantes EC, Souza GEP. Mediators involved in the febrile response induced by Tityus serrulatus scorpion venom in rats. Toxicon. 2006; 48:556–566.
Article25. Pinto MCL, Melo MM, Costa MER, Labarrére CR. Hematological and biochemical profiles of rats submitted to experimental poisoning with Tityus serrulatus venom. Arq Bras Med Vet Zootec. 2010; 62:350–356.
Article26. Possani LD. Structure of scorpion toxins. In : Tu AT, editor. Handbook of Natural Toxins: Insects Poisons, Allergens and Other Invertebrate Venoms. New York: Dekker;1984. p. 514–545.27. Possani LD, Alagón AC, Fletcher PL Jr, Erickson BW. Purification and properties of mammalian toxins from the venom of the Brazilian scorpion Tityus serrulatus Lutz and Mello. Arch Biochem Biophys. 1977; 180:394–403.
Article28. Pugh DG. Sheep and Goat Medicine. Philadelphia: Saunders;2002. p. 468.29. Ribeiro EL, Melo MM, Pinto MCL, Labarrère CR, Guimarães PTC, Paes PRO, Leme FOP. Canine blood profile after experimental envenomation by Tityus serrulatus. Arq Bras Med Vet Zootec. 2009; 61:135–143.30. Ribeiro EL, Melo MM, Silva EF, Melo MB, Labarrère CR, Merlo FA, Veado JCC. Cardiovascular and clinical evaluation of dogs after Tityus serrulatus venom experimentally inoculation. MedveP: Rev Cien Med Vet Pequeno Anim Anim Estim. 2010; 28:21–28.31. Sampaio IBM. Estatística Aplicada à Experimentação Animal. 3rd ed. Belo Horizonte: FEPMVZ-UFMG;2007. p. 264.32. Schultz RA, Pretorius PJ, Terblanche M. An electrocardiographic study of normal sheep using a modified technique. Onderstepoort J Vet Res. 1972; 39:97–106.33. Sjostrom L, al-Abdulla IH, Rawat S, Smith DC, Landon J. A comparison of ovine and equine antivenoms. Toxicon. 1994; 32:427–433.
Article34. Stills HF Jr. Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. ILAR J. 2005; 46:280–293.
Article35. Thrall MA. Veterinary Hematology and Clinical Chemistry. Philadelphia: Lippincott Williams & Wilkins;2003. p. 518.36. Tilley LP. Essentials of Canine and Feline Electrocardiography: Interpretation and Treatment. Philadelphia: Lea & Febiger;1992. p. 470.37. Torío R, Cano M, Montes A, Prieto F, Benedito JL. Comparison of two methods for electrocardiographic analysis in Gallega sheep. Small Rumin Res. 1997; 24:239–246.
Article38. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979; 76:4350–4354.
Article39. Uemitsu N, Kawasaki H, Furuhashi T, Miyoshi K, Ohtaka T, Nomura A, Hasegawa T, Shimizu Y, Nakazawa M. Acute and subacute toxicity studies and local irritation study of glutaraldehyde. Oyo Yakuri. 1976; 12:11–32.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Newly Developed Pemphigus Foliaceus and Possible Association with Alternative Bee-Venom Therapy
- Bee Venom Therapy for Musculoskeletal Disorders
- Evaluation of Antifungal Activities of Bee Venom Components Against Malassezia Strains
- A Case of Ischemic Stroke Following Bee Venom Acupuncture
- Detection of honeybee venom specific igE and igG4 in honeybee venom allergy