Ann Surg Treat Res.
2017 Jun;92(6):402-410. 10.4174/astr.2017.92.6.402.
Comparison of effects of the tacrolimus and cyclosporine A on the colon anastomosis recovery of rats
- Affiliations
-
- 1Department of General Surgery, Sanko University School of Medicine, Gaziantep, Turkey. drerdaluysal@hotmail.com
- 2Department of Emergency, Necip Fazil City Hospital, Kahramanmaras, Turkey.
- KMID: 2387413
- DOI: http://doi.org/10.4174/astr.2017.92.6.402
Abstract
- PURPOSE
This study aims to examine and compare the effects of immunosuppressant cyclosporine A (CsA) and tacrolimus (TAC) on colon anastomosis recovery.
METHODS
Forty rats were randomly divided into 4 groups. The 4 groups were determined as follows: control group; sham group, given %0.09 NaCl; TAC group, given 0.5 mg/kg/day tacrolimus; and CsA group, given 5 mg/kg/day CsA. A 6-cm midabdomen incision was performed on the rats. An incision of all layers on the right colon was performed. Then anastomosis was undertaken. Laparotomy was performed on the seventh day postoperation. The colon bursting pressures were evaluated, histopathological examinations were undertaken, and E-cadherin expression and tissue hydroxyproline levels were evaluated.
RESULTS
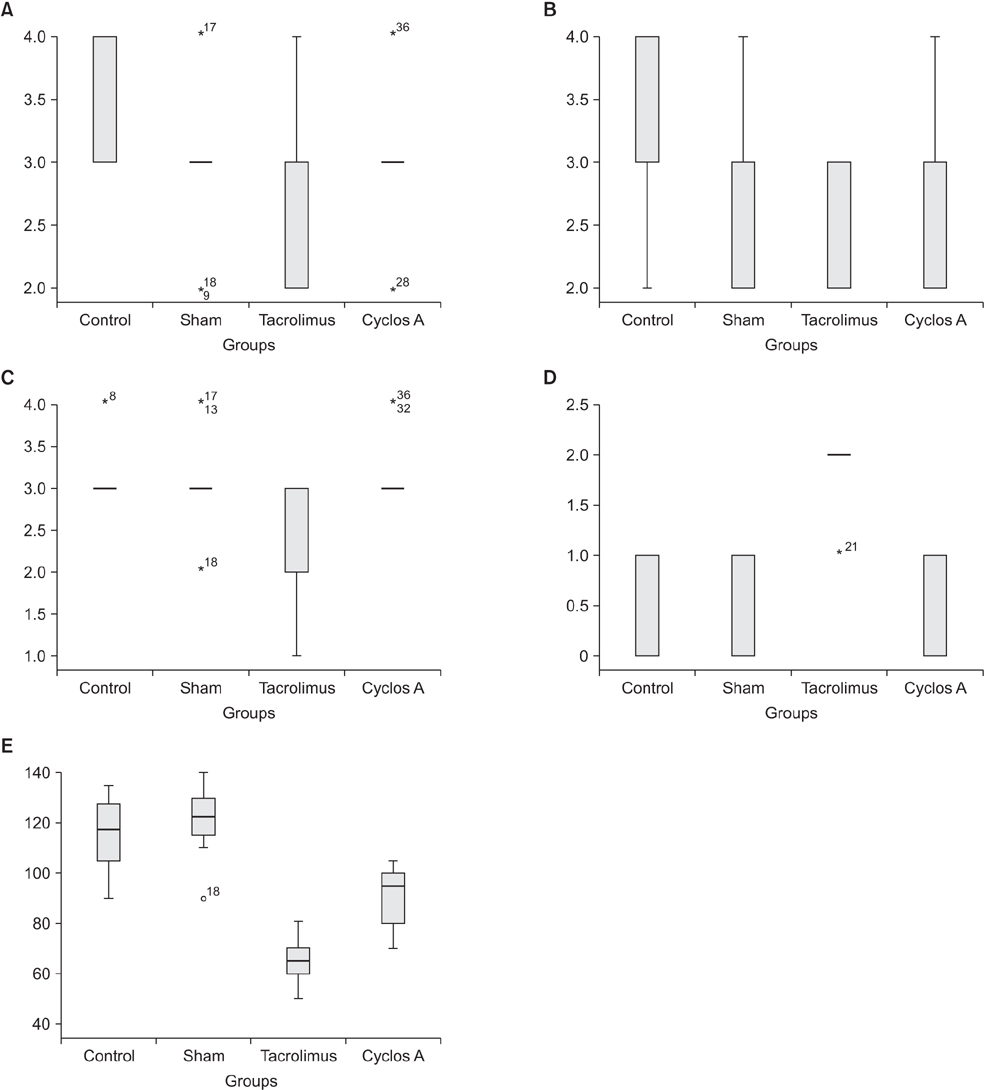
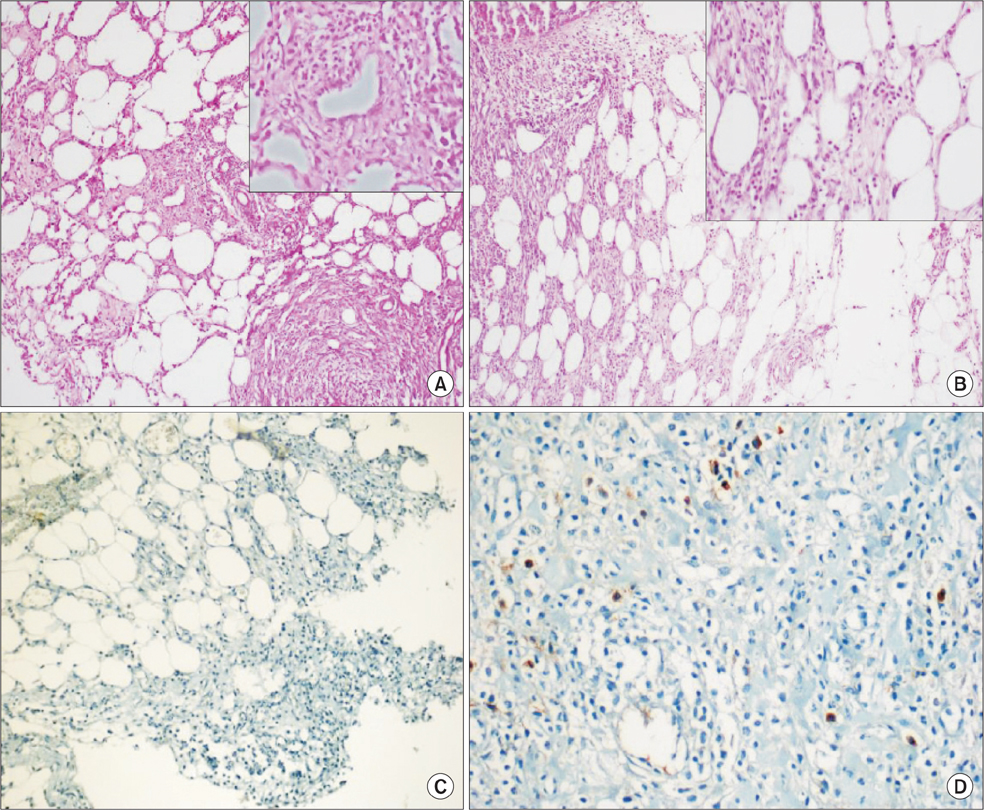
Statistically significant differences were observed among bursting pressures of the groups (P < 0.001). The value was significantly low in TAC and CsA groups when compared to control and sham groups (P < 0.05). The tissue hydroxyproline levels were significantly low in TAC group compared to control group (P = 0.03). Fibroblast density and neovascularization were significantly greater in the control group compared to the TAC group (P < 0.05). Levels of collagen had decreased significantly in TAC group compared to other groups (P < 0.05).
CONCLUSION
Our study showed that TAC may have a negative effect of colon anastomosis recovery. The lowest anastomosis bursting pressure was detected in TAC group. Also, collagen, hydroxyproline, fibroblast, neovascularization and E-Cadherin levels were comparatively lower in TAC group. CsA did not cause any significant changes to tissue hydroxyproline, collagen, fibroblast, and E-Cadherin levels.
Keyword
MeSH Terms
Figure
Reference
-
1. Willems MC, van der Vliet JA, Lomme RM, Hendriks T. Tacrolimus does not affect early wound healing in a rodent model of bowel anastomoses and abdominal wall closure. PLoS One. 2013; 8:e76348.2. Seyhun Y, Ciftci HS, Kekik C, Karadeniz MS, Tefik T, Nane I, et al. Genetic association of interleukin-2, interleukin-4, interleukin-6, transforming growth factor-β, tumour necrosis factor-α and blood concentrations of calcineurin inhibitors in Turkish renal transplant patients. Int J Immunogenet. 2015; 42:147–160.3. Chung BH, Kim KW, Kim BM, Piao SG, Lim SW, Choi BS, et al. Dysregulation of Th17 cells during the early post-transplant period in patients under calcineurin inhibitor based immunosuppression. PLoS One. 2012; 7:e42011.4. Kita J, Ogino Y, Kobayashi E, Fujimura A, Kogure H. Effects of tacrolimus on small and large bowel anastomoses in the rat. Transplant Proc. 1999; 31:2789.5. Willems MC, van der Vliet JA, de Man BM, van der Laak JA, Lomme RM, Hendriks T. Persistent effects of everolimus on strength of experimental wounds in intestine and fascia. Wound Repair Regen. 2010; 18:98–104.6. Karaca G, Pekcici MR, Altunkaya C, Fidanci V, Kilinc A, Ozer H, et al. The effects of scalpel, harmonic scalpel and monopolar electrocautery on the healing of colonic anastomosis after colonic resection. Ann Surg Treat Res. 2016; 90:315–321.7. Kedzierska K, Sindrewicz K, Sporniak-Tutak K, Bober J, Stanczyk-Dunaj M, Dolegowska B, et al. Effect of immunosuppressive therapy on proteinogram in rats. Med Sci Monit. 2016; 22:1987–1998.8. Kabat-Koperska J, Kolasa-Wolosiuk A, Wojciuk B, Wojciechowska-Koszko I, Roszkowska P, Krasnodebska-Szponder B, et al. The influence of intrauterine exposure to immunosuppressive treatment on changes in the immune system in juvenile Wistar rats. Drug Des Devel Ther. 2016; 10:2279–2288.9. Li Z, Sun F, Zhang Y, Chen H, He N, Chen H, et al. Tacrolimus induces insulin resistance and increases the glucose absorption in the jejunum: a potential mechanism of the diabetogenic effects. PLoS One. 2015; 10:e0143405.10. Ehrlich HP, Tarver H, Hunt TK. Effects of vitamin A and glucocorticoids upon inflammation and collagen synthesis. Ann Surg. 1973; 177:222–227.11. Tuncel H, Shimamoto F, Cagatay P, Kalkan MT. Variable E-cadherin expression in a MNU-induced colon tumor model in rats which exposed with 50 Hz frequency sinusoidal magnetic field. Tohoku J Exp Med. 2002; 198:245–249.12. Pramateftakis MG, Kanellos D, Demetriades H, Kanellos I, Mantzoros I, Zacharakis E, et al. The effects of irinotecan on the healing of colonic anastomoses in rats. Open Surg J. 2007; 1:1–6.13. Schaffer M, Fuchs N, Volker J, Schulz T, Kapischke M, Viebahn R. Differential effect of tacrolimus on dermal and intestinal wound healing. J Invest Surg. 2005; 18:71–79.14. Eisinger DR, Sheil AG. A comparison of the effects of cyclosporin A and standard agents on primary wound healing in the rat. Surg Gynecol Obstet. 1985; 160:135–138.15. Pinsker KL, Veith FJ, Kamholz SL, Emeson EE, Norin A, Montefusco C. Bronchial anastomotic healing in canine lung allotransplants treated with cyclosporine. Transplantation. 1985; 40:143–146.16. Kuper MA, Trutschel S, Weinreich J, Konigsrainer A, Beckert S. Growth hormone abolishes the negative effects of everolimus on intestinal wound healing. World J Gastroenterol. 2016; 22:4321–4329.17. Kanellos I, Mantzoros I, Demetriades H, Kalfadis S, Kelpis T, Sakkas L, et al. Healing of colon anastomoses covered with fibrin glue after immediate postoperative intraperitoneal administration of 5-fluorouracil. Dis Colon Rectum. 2004; 47:510–515.18. Halsted W. Circular suture of the intestine an experimental study. Am J Med Sci. 1887; 94:436–461.19. Savage FJ, Lacombe DL, Boulos PB, Hembry RM. Role of matrix metalloproteinases in healing of colonic anastomosis. Dis Colon Rectum. 1997; 40:962–970.20. Pronio A, Di Filippo A, Narilli P, Mancini B, Caporilli D, Piroli S, et al. Anastomotic dehiscence in colorectal surgery. Analysis of 1290 patients. Chir Ital. 2007; 59:599–609.21. Brunner G. Theme issue: Wound healing mechanisms. Thromb Haemost. 2003; 90:976–977.22. Raptis D, Mantzoros I, Pramateftakis MG, Despoudi K, Zaraboukas T, Koliakos G, et al. The effects of tacrolimus on colonic anastomotic healing in rats. Int J Colorectal Dis. 2012; 27:299–308.23. Turgut B, Guler M, Akpolat N, Demır T, Celıker U. The impact of tacrolimus on vascular endothelial growth factor in experimental corneal neovascularization. Curr Eye Res. 2011; 36:34–40.24. Uzunkoy A, Akinci OF, Coskun A, Aslan O, Kocyigit A. Effects of antiadhesive agents on the healing of intestinal anastomosis. Dis Colon Rectum. 2000; 43:370–375.25. Clark RA, Nielsen LD, Welch MP, McPherson JM. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. J Cell Sci. 1995; 108(Pt 3):1251–1261.26. Que J, Cao Q, Sui T, Du S, Kong D, Cao X. Effect of FK506 in reducing scar formation by inducing fibroblast apoptosis after sciatic nerve injury in rats. Cell Death Dis. 2013; 4:e526.27. Janikowska G, Janikowsk T, Pyka A, Wilczok A, Mazurek U. Cyclosporin a affects the proliferation process in normal human dermal fibroblasts. Acta Pol Pharm. 2016; 73:55–63.28. Cheong YC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001; 7:556–566.29. Pastor-Clerigues A, Serrano A, Milara J, Marti-Bonmati E, Lopez-Perez FJ, Garcia-Montanes S, et al. Evaluation of the ocular tolerance of three tacrolimus topical pharmaceutical preparations by bovine corneal opacity and permeability test. Curr Eye Res. 2016; 41:890–896.30. Cohen SR, Cornell CN, Collins MH, Sell JE, Blanc WA, Altman RP. Healing of ischemic colonic anastomoses in the rat: role of antibiotic preparation. Surgery. 1985; 97:443–446.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chronic urticaria treated with tacrolimus
- Comparison of Tacrolimus and Cyclosporine in Kidney Transplantation
- A Case of Recalcitrant Psoriasis Improved with Tacrolimus (FK 506)
- Effect of Immunosuppressive Drugs on the Metalloproteinase in the Glioma Cells and Osteoblasts
- A Case of Papuloerythroderma of Ofuji Improved by Cyclosporine and Topical Tacrolimus