Yonsei Med J.
2014 Mar;55(2):467-475.
Risk Factors and Molecular Epidemiology of Community-Onset Extended-Spectrum beta-Lactamase-Producing Escherichia coli Bacteremia
- Affiliations
-
- 1Department of Internal Medicine, Gachon University, Gil Medical Center, Incheon, Korea. karmacho@gilhospital.com
- 2Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea. kscpjsh@yuhs.ac
- 3Department of Laboratory Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Department of Social and Preventive Medicine, Inha University School of Medicine, Incheon, Korea.
- 5Department of Laboratory Medicine, Gachon University, Gil Medical Center, Incheon, Korea.
- 6Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
Inadequate empirical therapy for severe infections caused by extended-spectrum beta-lactamase-producing Escherichia coli (ESBLEC) is associated with poor outcomes. This study was designed to investigate risk factors for community-onset ESBLEC bacteremia at admission to a tertiary care hospital.
MATERIALS AND METHODS
A case-control study was performed that included all episodes of ESBLEC bacteremia in the outpatient department or within 48 hours of admission from January 2005 to March 2009. Data on predisposing factors were collected. The molecular epidemiology of ESBLEC clinical isolates was also determined.
RESULTS
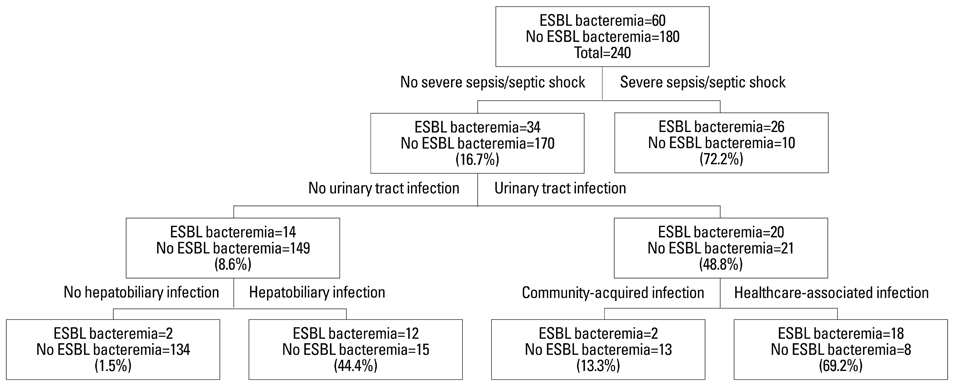
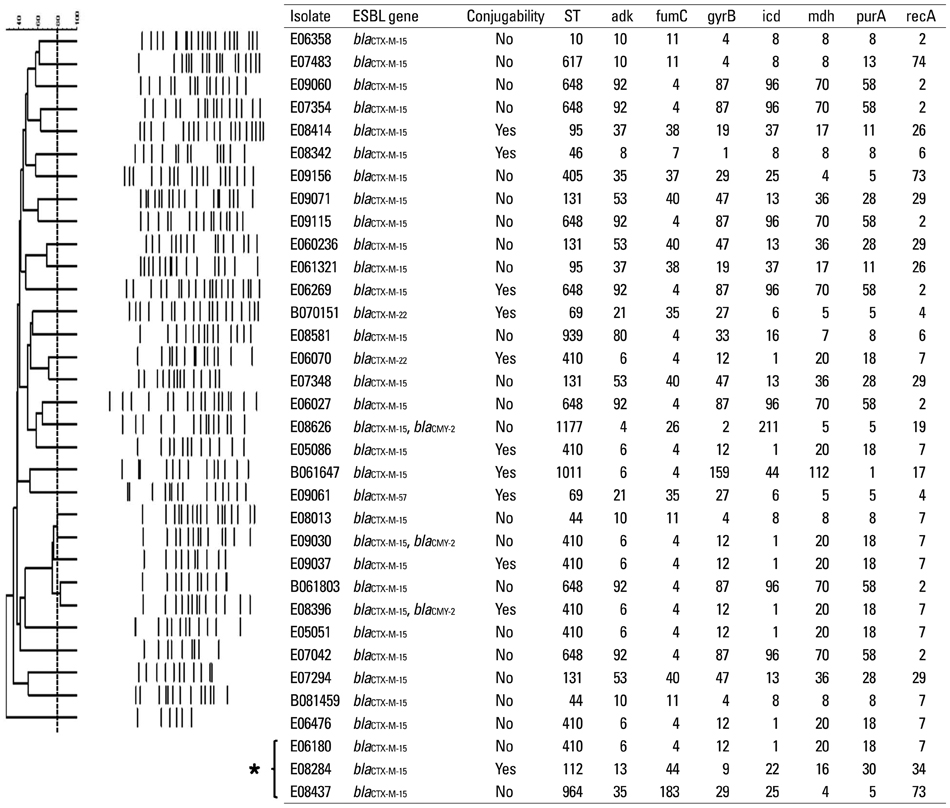
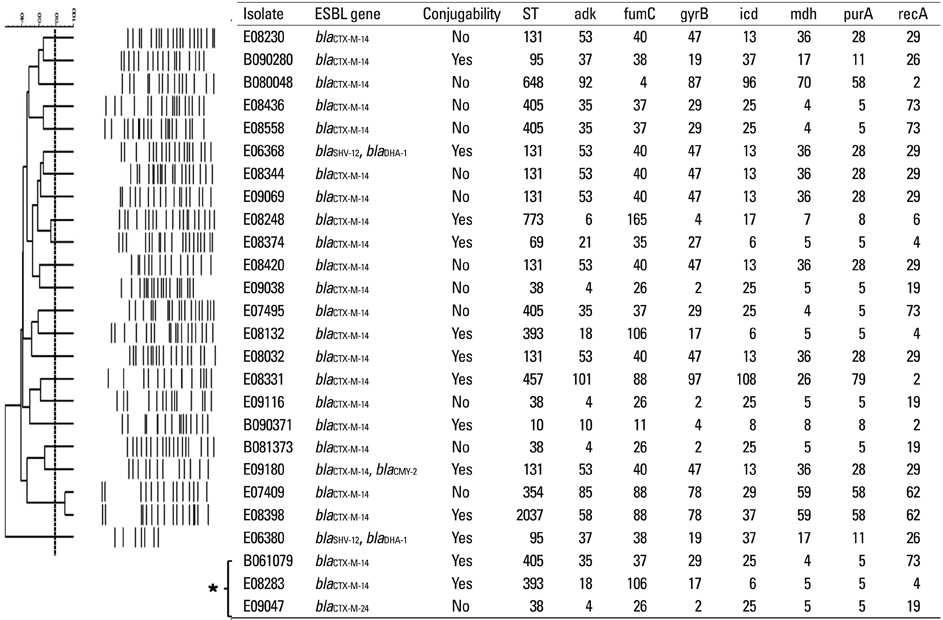
Among 25281 blood cultures, 60 episodes of ESBLEC bacteremia were studied, which accounted for 7% of all E. coli bacteremia at admission. Healthcare-associated infection [odds ratio (OR), 8.3; 95% confidence interval (CI), 2.4-28.7; p=0.001], malignancy (OR, 4.6; 95% CI, 1.3-16.3; p=0.018), urinary tract infection (OR, 139.1; 95% CI, 24.6-788.2; p<0.001), hepatobiliary infection (OR, 79.1; 95% CI, 13.5-463.8; p<0.001), third generation cephalosporin usage during preceding 3 months (OR, 16.4; 95% CI, 2.0-131.8; p=0.008), and severe sepsis/septic shock (OR, 73.7; 95% CI, 12.4-438.5; p<0.001) were determined as independent risk factors for community-onset ESBLEC bacteremia. The most common extended-spectrum beta-lactamase (ESBL) gene identified was bla(CTX-M-15) (n=31) followed by bla(CTX-M-14) (n=23).
CONCLUSION
The most common types of ESBLs in E. coli causing community-onset bacteremia were CTX-M-15 and CTX-M-14 in Korea. By result of decision tree analysis, the empirical use of carbapenems is suggested only for patients with severe sepsis/septic shock, hepatobiliary infection, or healthcare-associated urinary tract infection.
Keyword
MeSH Terms
Figure
Reference
-
1. Pitout JD, Nordmann P, Laupland KB, Poirel L. Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J Antimicrob Chemother. 2005; 56:52–59.
Article2. Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001; 32:1162–1171.
Article3. Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007; 51:1987–1994.
Article4. Rodríguez-Baño J, Navarro MD, Romero L, Muniain MA, de Cueto M, Ríos MJ, et al. Bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli in the CTX-M era: a new clinical challenge. Clin Infect Dis. 2006; 43:1407–1414.5. Hyle EP, Lipworth AD, Zaoutis TE, Nachamkin I, Bilker WB, Lautenbach E. Impact of inadequate initial antimicrobial therapy on mortality in infections due to extended-spectrum beta-lactamase-producing enterobacteriaceae: variability by site of infection. Arch Intern Med. 2005; 165:1375–1380.
Article6. Rossolini GM, D'Andrea MM, Mugnaioli C. The spread of CTX-M-type extended-spectrum beta-lactamases. Clin Microbiol Infect. 2008; 14:Suppl 1. 33–41.7. Ryoo NH, Kim EC, Hong SG, Park YJ, Lee K, Bae IK, et al. Dissemination of SHV-12 and CTX-M-type extended-spectrum beta-lactamases among clinical isolates of Escherichia coli and Klebsiella pneumoniae and emergence of GES-3 in Korea. J Antimicrob Chemother. 2005; 56:698–702.
Article8. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373–383.
Article9. Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med. 2004; 140:26–32.
Article10. Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002; 137:791–797.
Article11. Cockerill FR. Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement. Wayne, PA: CLSI;2010.12. Song W, Jeong SH, Kim JS, Kim HS, Shin DH, Roh KH, et al. Use of boronic acid disk methods to detect the combined expression of plasmid-mediated AmpC beta-lactamases and extended-spectrum beta-lactamases in clinical isolates of Klebsiella spp., Salmonella spp., and Proteus mirabilis. Diagn Microbiol Infect Dis. 2007; 57:315–318.
Article13. Jacoby GA, Han P. Detection of extended-spectrum beta-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol. 1996; 34:908–911.
Article14. Anderson DJ, Engemann JJ, Harrell LJ, Carmeli Y, Reller LB, Kaye KS. Predictors of mortality in patients with bloodstream infection due to ceftazidime-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2006; 50:1715–1720.
Article15. Peña C, Gudiol C, Calatayud L, Tubau F, Domínguez MA, Pujol M, et al. Infections due to Escherichia coli producing extended-spectrum beta-lactamase among hospitalised patients: factors influencing mortality. J Hosp Infect. 2008; 68:116–122.
Article16. Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003; 115:529–535.
Article17. Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005; 49:760–766.
Article18. Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009; 136:1237–1248.
Article19. Rodríguez-Baño J, Navarro MD, Romero L, Muniain MA, Cueto Md, Gálvez J, et al. Risk-factors for emerging bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Clin Microbiol Infect. 2008; 14:180–183.
Article20. Ho PL, Chan WM, Tsang KW, Wong SS, Young K. Bacteremia caused by Escherichia coli producing extended-spectrum beta-lactamase: a case-control study of risk factors and outcomes. Scand J Infect Dis. 2002; 34:567–573.
Article21. Rodríguez-Baño J, Picón E, Gijón P, Hernández JR, Ruíz M, Peña C, et al. Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis. 2010; 50:40–48.
Article22. Kang CI, Song JH, Chung DR, Peck KR, Ko KS, Yeom JS, et al. Risk factors and treatment outcomes of community-onset bacteraemia caused by extended-spectrum beta-lactamase-producing Escherichia coli. Int J Antimicrob Agents. 2010; 36:284–287.
Article23. Gudiol C, Calatayud L, Garcia-Vidal C, Lora-Tamayo J, Cisnal M, Duarte R, et al. Bacteraemia due to extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother. 2010; 65:333–341.
Article24. Kaye KS, Harris AD, Samore M, Carmeli Y. The case-case-control study design: addressing the limitations of risk factor studies for antimicrobial resistance. Infect Control Hosp Epidemiol. 2005; 26:346–351.
Article25. Harris AD, Karchmer TB, Carmeli Y, Samore MH. Methodological principles of case-control studies that analyzed risk factors for antibiotic resistance: a systematic review. Clin Infect Dis. 2001; 32:1055–1061.
Article26. Harris AD, Samore MH, Carmeli Y. Control group selection is an important but neglected issue in studies of antibiotic resistance. Ann Intern Med. 2000; 133:159.
Article27. Gerald LB, Tang S, Bruce F, Redden D, Kimerling ME, Brook N, et al. A decision tree for tuberculosis contact investigation. Am J Respir Crit Care Med. 2002; 166:1122–1127.
Article28. Kammerer JS, McNabb SJ, Becerra JE, Rosenblum L, Shang N, Iademarco MF, et al. Tuberculosis transmission in nontraditional settings: a decision-tree approach. Am J Prev Med. 2005; 28:201–207.29. Temkin NR, Holubkov R, Machamer JE, Winn HR, Dikmen SS. Classification and regression trees (CART) for prediction of function at 1 year following head trauma. J Neurosurg. 1995; 82:764–771.
Article30. Kvasnicka HM, Thiele J, Schmitt-Graeff A, Diehl V, Zankovich R, Niederle N, et al. Bone marrow features improve prognostic efficiency in multivariate risk classification of chronic-phase Ph(1+) chronic myelogenous leukemia: a multicenter trial. J Clin Oncol. 2001; 19:2994–3009.
Article31. Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ. ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005; 293:572–580.
Article32. Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med. 2012; 27:128–142.
Article33. Kim SY, Shin J, Shin SY, Ko KS. Characteristics of carbapenem-resistant Enterobacteriaceae isolates from Korea. Diagn Microbiol Infect Dis. 2013; 76:486–490.
Article34. Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008; 168:1897–1902.
Article35. Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008; 8:159–166.
Article36. Peirano G, Pitout JD. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4. Int J Antimicrob Agents. 2010; 35:316–321.
Article37. Song W, Lee H, Lee K, Jeong SH, Bae IK, Kim JS, et al. CTX-M-14 and CTX-M-15 enzymes are the dominant type of extended-spectrum beta-lactamase in clinical isolates of Escherichia coli from Korea. J Med Microbiol. 2009; 58(Pt 2):261–266.
Article38. Kang CI, Wi YM, Lee MY, Ko KS, Chung DR, Peck KR, et al. Epidemiology and risk factors of community onset infections caused by extended-spectrum β-lactamase-producing Escherichia coli strains. J Clin Microbiol. 2012; 50:312–317.
Article39. Park SH, Byun JH, Choi SM, Lee DG, Kim SH, Kwon JC, et al. Molecular epidemiology of extended-spectrum β-lactamase-producing Escherichia coli in the community and hospital in Korea: emergence of ST131 producing CTX-M-15. BMC Infect Dis. 2012; 12:149.
Article40. Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis. 2010; 51:286–294.
Article41. Coque TM, Novais A, Carattoli A, Poirel L, Pitout J, Peixe L, et al. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg Infect Dis. 2008; 14:195–200.
Article42. Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, Caniça MM, et al. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother. 2008; 61:273–281.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Community-onset Necrotizing Fasciitis Caused by Extended-spectrum Beta-lactamase-producing Eshcerichia coli
- Risk Factors of Nosocomial Bacteremia of Extended-spectrum beta-Lactamase Producing Escherichia coli
- A Case Report of Sepsis by Extended-Spectrum beta-Lactamase Producing Escherichia Coli
- Community-Onset Extended-Spectrum Beta-Lactamase-Producing Escherichia coli Osteomyelitis of the Pubic Symphysis
- In Vitro Susceptibility of piperacillin/tazobactam Against extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae