Yonsei Med J.
2014 Mar;55(2):331-338.
Diagnostic Algorithm to Reflect Regressive Changes of Human Papilloma Virus in Tissue Biopsies
- Affiliations
-
- 1Life-Care Research Center, Samsung Insurance Comp., Seoul, Korea.
- 2Department of Pathology, Yonsei University College of Medicine, Seoul, Korea. cho1988@yuhs.ac
- 3Bommedical Co., Ltd., Seoul, Korea.
- 4Department of Gynecologic Oncology, Yonsei University College of Medicine, Seoul, Korea.
- 5Biomedical Institute, Yonsei University College of Medicine, Seoul, Korea.
- 6Brain Korea 21 Project for Medical Science, Seoul, Korea.
Abstract
- PURPOSE
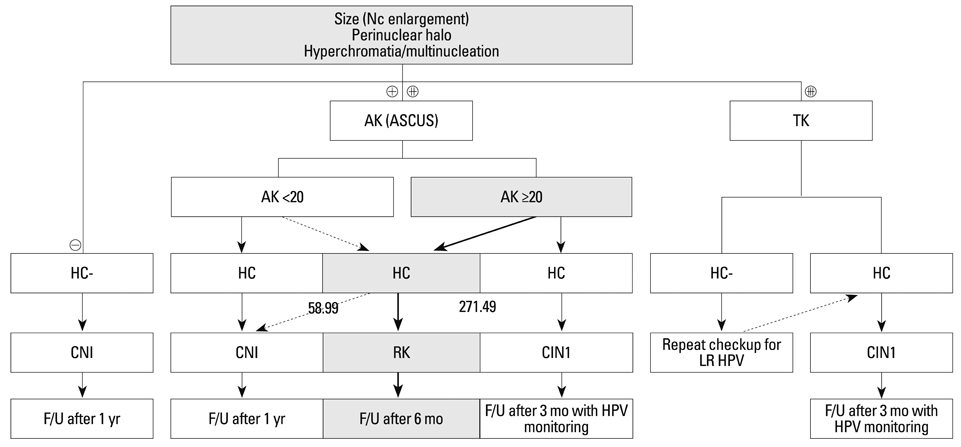
Landmark indicators have not yet to be developed to detect the regression of cervical intraepithelial neoplasia (CIN). We propose that quantitative viral load and indicative histological criteria can be used to differentiate between atypical squamous cells of undetermined significance (ASCUS) and a CIN of grade 1.
MATERIALS AND METHODS
We collected 115 tissue biopsies from women who tested positive for the human papilloma virus (HPV). Nine morphological parameters including nuclear size, perinuclear halo, hyperchromasia, typical koilocyte (TK), abortive koilocyte (AK), bi-/multi-nucleation, keratohyaline granules, inflammation, and dyskeratosis were examined for each case. Correlation analyses, cumulative logistic regression, and binary logistic regression were used to determine optimal cut-off values of HPV copy numbers. The parameters TK, perinuclear halo, multi-nucleation, and nuclear size were significantly correlated quantitatively to HPV copy number.
RESULTS
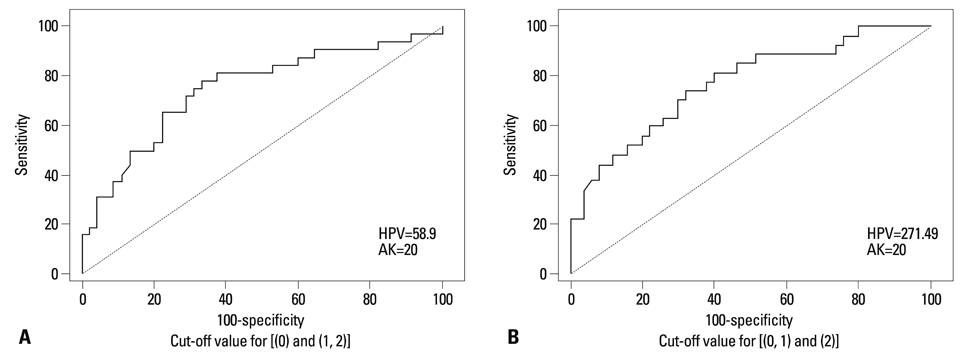
An HPV loading number of 58.9 and AK number of 20 were optimal to discriminate between negative and subtle findings in biopsies. An HPV loading number of 271.49 and AK of 20 were optimal for discriminating between equivocal changes and obvious koilocytosis.
CONCLUSION
We propose that a squamous epithelial lesion with AK of >20 and quantitative HPV copy number between 58.9-271.49 represents a new spectrum of subtle pathological findings, characterized by AK in ASCUS. This can be described as a distinct entity and called "regressing koilocytosis".
Keyword
MeSH Terms
Figure
Reference
-
1. Kulmala SM, Syrjänen S, Shabalova I, Petrovichev N, Kozachenko V, Podistov J, et al. Human papillomavirus testing with the hybrid capture 2 assay and PCR as screening tools. J Clin Microbiol. 2004; 42:2470–2475.
Article2. Ratnam S, Franco EL, Ferenczy A. Human papillomavirus testing for primary screening of cervical cancer precursors. Cancer Epidemiol Biomarkers Prev. 2000; 9:945–951.3. Venturoli S, Cricca M, Bonvicini F, Giosa F, Pulvirenti FR, Galli C, et al. Human papillomavirus DNA testing by PCR-ELISA and hybrid capture II from a single cytological specimen: concordance and correlation with cytological results. J Clin Virol. 2002; 25:177–185.
Article4. Tsiodras S, Georgoulakis J, Chranioti A, Voulgaris Z, Psyrri A, Tsivilika A, et al. Hybrid capture vs. PCR screening of cervical human papilloma virus infections. Cytological and histological associations in 1270 women. BMC Cancer. 2010; 10:53.
Article5. Clavel C, Masure M, Levert M, Putaud I, Mangeonjean C, Lorenzato M, et al. Human papillomavirus detection by the hybrid capture II assay: a reliable test to select women with normal cervical smears at risk for developing cervical lesions. Diagn Mol Pathol. 2000; 9:145–150.
Article6. Ylitalo N, Sørensen P, Josefsson AM, Magnusson PK, Andersen PK, Pontén J, et al. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case-control study. Lancet. 2000; 355:2194–2198.
Article7. Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998; 338:423–428.
Article8. Tsai HT, Wu CH, Lai HL, Li RN, Tung YC, Chuang HY, et al. Association between quantitative high-risk human papillomavirus DNA load and cervical intraepithelial neoplasm risk. Cancer Epidemiol Biomarkers Prev. 2005; 14(11 Pt 1):2544–2549.
Article9. Knoepp SM, Kuebler DL, Wilbur DC. Correlation between hybrid capture II high-risk human papillomavirus DNA test chemiluminescence intensity from cervical samples with follow-up histologic results: a cytologic/histologic review of 367 cases. Cancer Cytopathol. 2010; 118:209–217.
Article10. Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee SK, Kuypers JM, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005; 191:731–738.
Article11. Wentzensen N, Wilson LE, Wheeler CM, Carreon JD, Gravitt PE, Schiffman M, et al. Hierarchical clustering of human papilloma virus genotype patterns in the ASCUS-LSIL triage study. Cancer Res. 2010; 70:8578–8586.
Article12. Bollmann M, Bánkfalvi A, Trosic A, Speich N, Schmittt C, Bollmann R. Can we detect cervical human papillomavirus (HPV) infection by cytomorphology alone? Diagnostic value of non-classic cytological signs of HPV effect in minimally abnormal Pap tests. Cytopathology. 2005; 16:13–21.
Article13. Nijhawan R, Mittal N, Suri V, Rajwanshi A. Enhancing the scope of conventional cervical cytology for detecting HPV infection. Diagn Cytopathol. 2010; 38:645–651.
Article14. An HJ, Sung JM, Park AR, Song KJ, Lee YN, Kim YT, et al. Prospective evaluation of longitudinal changes in human papillomavirus genotype and phylogenetic clade associated with cervical disease progression. Gynecol Oncol. 2011; 120:284–290.
Article15. Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010; 117:2 Suppl. S5–S10.
Article16. Roteli-Martins CM, Alves VA, Santos RT, Martinez EZ, Syrjänen KJ, Derchain SF. Value of morphological criteria in diagnosing cervical HPV lesions confirmed by in situ hybridization and hybrid capture assay. Pathol Res Pract. 2001; 197:677–682.
Article17. Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS. 2010; 118:422–449.
Article18. Krawczyk E, Suprynowicz FA, Liu X, Dai Y, Hartmann DP, Hanover J, et al. Koilocytosis: a cooperative interaction between the human papillomavirus E5 and E6 oncoproteins. Am J Pathol. 2008; 173:682–688.19. Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010; 10:550–560.
Article20. Huh WK. Human papillomavirus infection: a concise review of natural history. Obstet Gynecol. 2009; 114:139–143.21. Lawson JS, Glenn WK, Heng B, Ye Y, Tran B, Lutze-Mann L, et al. Koilocytes indicate a role for human papilloma virus in breast cancer. Br J Cancer. 2009; 101:1351–1356.
Article22. Sherman ME, Wang SS, Wheeler CM, Rich L, Gravitt PE, Tarone R, et al. Determinants of human papillomavirus load among women with histological cervical intraepithelial neoplasia 3: dominant impact of surrounding low-grade lesions. Cancer Epidemiol Biomarkers Prev. 2003; 12:1038–1044.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to “Diagnostic Algorithm to Reflect Regressive Changes of Human Papilloma Virus in Tissue Biopsies†by Lhee MJ, et al. (Yonsei Med J 2014;55:331-338.)
- Complete Remission of Recalcitrant Plantar Wart Treated with Quadrivalent Human Papilloma Virus Vaccine
- Human Papilloma Virus Positive Oropharyngeal Cancer
- Detection of Human Papillomavirus DNA in Genital and Laryngeal Papilloma Using the Polymerase Chain Reaction
- Immunohistochemical study on the expression of matrix metalloproteinase 2 and high-risk human papilloma virus in the malignant progression of papillomas