Endocrinol Metab.
2014 Dec;29(4):450-456. 10.3803/EnM.2014.29.4.450.
Using Growth Hormone Levels to Detect Macroadenoma in Patients with Acromegaly
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Endocrinology and Metabolism, Gachon University Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea. kwkim@gilhospital.com
- KMID: 2384241
- DOI: http://doi.org/10.3803/EnM.2014.29.4.450
Abstract
- BACKGROUND
The aim of this study was to assess the clinical differences between acromegalic patients with microadenoma and patients with macroadenoma, and to evaluate the predictive value of growth hormone (GH) levels for early detection of macroadenoma.
METHODS
We performed a retrospective analysis of 215 patients diagnosed with a GH-secreting pituitary adenoma. The patients were divided into two groups: the microadenoma group and the macroadenoma group, and the clinical parameters were compared between these two groups. The most sensitive and specific GH values for predicting macroadenoma were selected using receiver operating characteristic (ROC) curves.
RESULTS
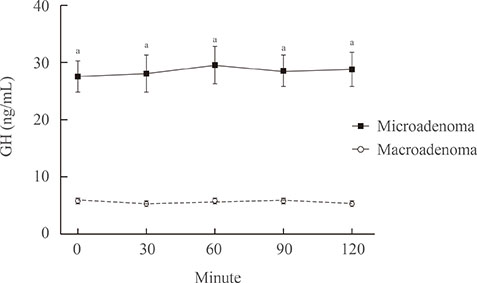
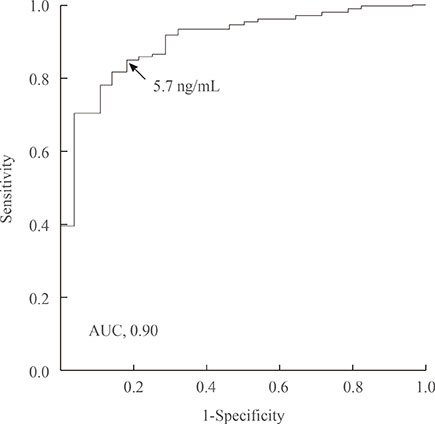
Compared with the microadenoma group, the macroadenoma group had a significantly younger age, higher body mass index, higher prevalence of hyperprolactinemia and hypogonadism, and a lower proportion of positive suppression to octreotide. However, there were no significant differences in the gender or in the prevalence of diabetes between the two groups. The tumor diameter was positively correlated with all GH values during the oral glucose tolerance test (OGTT). All GH values were significantly higher in the macroadenoma group than the microadenoma group. Cut-off values for GH levels at 0, 30, 60, 90, and 120 minutes for optimal discrimination between macroadenoma and microadenoma were 5.6, 5.7, 6.3, 6.0, and 5.8 ng/mL, respectively. ROC curve analysis revealed that the GH value at 30 minutes had the highest area under the curve.
CONCLUSION
The GH level of 5.7 ng/mL or higher at 30 minutes during OGTT could provide sufficient information to detect macroadenoma at the time of diagnosis.
MeSH Terms
Figure
Reference
-
1. Melmed S. Medical progress: acromegaly. N Engl J Med. 2006; 355:2558–2573.2. Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical 'cure'. Eur J Endocrinol. 2005; 152:379–387.3. Swearingen B, Barker FG 2nd, Katznelson L, Biller BM, Grinspoon S, Klibanski A, Moayeri N, Black PM, Zervas NT. Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J Clin Endocrinol Metab. 1998; 83:3419–3426.4. Petersenn S, Buchfelder M, Gerbert B, Franz H, Quabbe HJ, Schulte HM, Grussendorf M, Reincke M. Participants of the German Acromegaly Register. Age and sex as predictors of biochemical activity in acromegaly: analysis of 1,485 patients from the German Acromegaly Register. Clin Endocrinol (Oxf). 2009; 71:400–405.5. Rieger A, Rainov NG, Ebel H, Sanchin L, Shibib K, Helfrich C, Hoffmann O, Burkert W. Factors predicting pituitary adenoma invasiveness in acromegalic patients. Neurosurg Rev. 1997; 20:182–187.6. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003; 26:Suppl 1. S5–S20.7. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.8. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000; 85:2402–2410.9. Etxabe J, Gaztambide S, Latorre P, Vazquez JA. Acromegaly: an epidemiological study. J Endocrinol Invest. 1993; 16:181–187.10. Nabarro JD. Acromegaly. Clin Endocrinol (Oxf). 1987; 26:481–512.11. Mercado M, Espinosa de los Monteros AL, Sosa E, Cheng S, Mendoza V, Hernandez I, Sandoval C, Guinto G, Molina M. Clinical-biochemical correlations in acromegaly at diagnosis and the real prevalence of biochemically discordant disease. Horm Res. 2004; 62:293–299.12. Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004; 25:102–152.13. Choi YJ, Kim HC, Kim HM, Park SW, Kim J, Kim DJ. Prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998-2005. Diabetes Care. 2009; 32:2016–2020.14. Clemmons DR, Van Wyk JJ, Ridgway EC, Kliman B, Kjellberg RN, Underwood LE. Evaluation of acromegaly by radioimmunoassay of somatomedin-C. N Engl J Med. 1979; 301:1138–1142.15. Rodrigues TC, Costenaro F, Fedrizzi D, Oliveira MD, Lima PB, Boschi V, Czepielewski MA. Diabetes mellitus in a cohort of patients with acromegaly. Arq Bras Endocrinol Metabol. 2011; 55:714–719.16. Nielsen JH, Galsgaard ED, Moldrup A, Friedrichsen BN, Billestrup N, Hansen JA, Lee YC, Carlsson C. Regulation of beta-cell mass by hormones and growth factors. Diabetes. 2001; 50:Suppl 1. S25–S29.17. Kasayama S, Otsuki M, Takagi M, Saito H, Sumitani S, Kouhara H, Koga M, Saitoh Y, Ohnishi T, Arita N. Impaired beta-cell function in the presence of reduced insulin sensitivity determines glucose tolerance status in acromegalic patients. Clin Endocrinol (Oxf). 2000; 52:549–555.18. Melmed S, Ho K, Klibanski A, Reichlin S, Thorner M. Clinical review 75: recent advances in pathogenesis, diagnosis, and management of acromegaly. J Clin Endocrinol Metab. 1995; 80:3395–3402.19. Asa SL, Ezzat S. The pathogenesis of pituitary tumours. Nat Rev Cancer. 2002; 2:836–849.20. Colao A, De Rosa M, Pivonello R, Balestrieri A, Cappabianca P, Di Sarno A, Rochira V, Carani C, Lombardi G. Short-term suppression of GH and IGF-I levels improves gonadal function and sperm parameters in men with acromegaly. J Clin Endocrinol Metab. 2002; 87:4193–4197.21. Bates AS, Evans AJ, Jones P, Clayton RN. Assessment of GH status in acromegaly using serum growth hormone, serum insulin-like growth factor-1 and urinary growth hormone excretion. Clin Endocrinol (Oxf). 1995; 42:417–423.22. Colao A, Pivonello R, Auriemma RS, Briganti F, Galdiero M, Tortora F, Caranci F, Cirillo S, Lombardi G. Predictors of tumor shrinkage after primary therapy with somatostatin analogs in acromegaly: a prospective study in 99 patients. J Clin Endocrinol Metab. 2006; 91:2112–2118.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diabetic Ketoacidosis in a Patient with Acromegaly
- A Case of Acromegaly Presenting with Diabetic Ketoacidosis
- A Case of Acromegaly with Gall Bladder Cancer
- Acromegaly with Normal Insulin-Like Growth Factor-1 Levels and Congestive Heart Failure as the First Clinical Manifestation
- A case of acromegaly with empty sella syndrome associated with colonic neoplasm