Allergy Asthma Immunol Res.
2017 Sep;9(5):446-452. 10.4168/aair.2017.9.5.446.
Efficacy of Nasal Cellulose Powder in the Symptomatic Treatment of Allergic Rhinitis: A Randomized, Double-Blind, Placebo-Controlled Trial
- Affiliations
-
- 1Division of Allergy and Immunology, Department of Pediatrics, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. mwiparat@hotmail.com
- KMID: 2383993
- DOI: http://doi.org/10.4168/aair.2017.9.5.446
Abstract
- PURPOSE
Nasal Cellulose Powder (NCP), which can prevent from binding an allergen to nasal mucosa, may reduce allergic rhinitis (AR) symptoms in dust mite-sensitized children. This study was conducted to assess the efficacy of NCP in improving clinical symptoms of a nasal airflow limitation and the response of nasal inflammatory cells.
METHODS
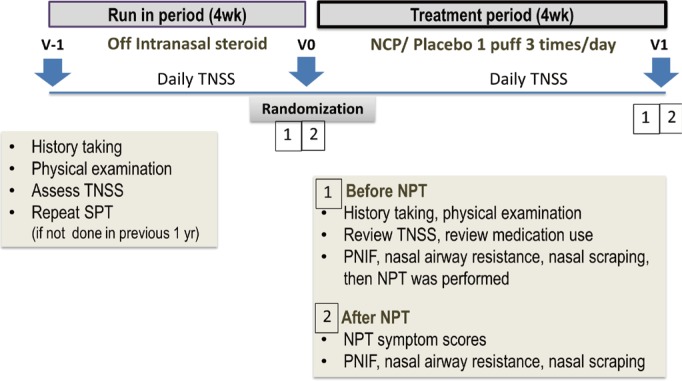
Children with dust mite-sensitized AR aged 6-18 years were recruited. After a 4-week run-in period, NCP or a placebo was administered, 1 puff per nostril 3 times daily for 4 weeks. The nasal provocation test (NPT) with Dermatophagoides pteronyssinus (Der p) was performed before and after treatment. The daily symptom scores (DSS), daily medication scores (DMS), the peak nasal inspiratory flows (PNIF), nasal airway resistance (NAR), as well as the maximum tolerated dose of NPT and eosinophil counts in nasal scraping, were evaluated.
RESULTS
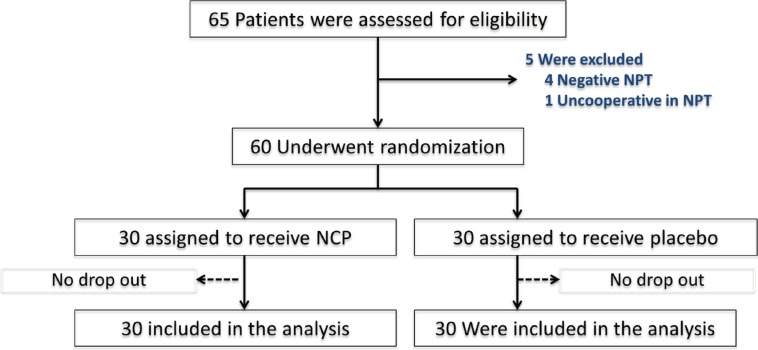
Sixty children (30 NCP and 30 placebos) were enrolled. Before treatment, there were no significant differences in age, dust mite control measures, DSS, DMS, PNIF, NAR, the maximum tolerated dose of NPT, or nasal eosinophil scores between children receiving NCP and placebos. After treatment, there were no significant differences between the NCP and placebo groups in the median (range) of the outcomes"”DSS: 2.06 (0.18-3.77) vs. 1.79 (0.08-7.79), P=0.756; DMS: 1.60 (0-5.13) vs. 0.56 (0-4.84), P=0.239; PNIF (L/min): 110 (60-160) vs. 100 (50-180), P=0.870; NAR (Pa/cm³/s): 0.40 (0.20-0.97) vs. 0.39 (0.24-1.32), P=0.690; the maximum tolerated dose of NPT and the nasal eosinophil scores: 1 (0-4) vs. 1 (0-4), P=0.861.
CONCLUSIONS
NCP treatment may not be more effective than placebo treatment in dust mite-sensitized AR children.
MeSH Terms
Figure
Cited by 1 articles
-
Benefits of Nasal Cellulose Powder Application Depend on the Type of Allergen Sensitization in Allergic Rhinitis
Todor A. Popov, Jean Emberlin, Nils Åberg, Peter Josling, Martin Church
Allergy Asthma Immunol Res. 2018;10(2):182-183. doi: 10.4168/aair.2018.10.2.182.
Reference
-
1. Bousquet J, Reid J, van Weel C, Baena Cagnani C, Canonica GW, Demoly P, et al. Allergic rhinitis management pocket reference 2008. Allergy. 2008; 63:990–996. PMID: 18691301.
Article2. Sritipsukho P. Aeroallergen sensitivity among Thai children with allergic respiratory diseases: a hospital-based study. Asian Pac J Allergy Immunol. 2004; 22:91–95. PMID: 15565944.3. Han DH, Ahn JC, Mun SJ, Park SK, Oh SY, Rhee CS. Novel risk factors for allergic rhinitis in Korean elementary school children: ARCO-kids phase II in a community. Allergy Asthma Immunol Res. 2015; 7:234–240. PMID: 25749770.
Article4. Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010; 126:466–476. PMID: 20816182.5. Portnoy J, Miller JD, Williams PB, Chew GL, Miller JD, Zaitoun F, et al. Environmental assessment and exposure control of dust mites: a practice parameter. Ann Allergy Asthma Immunol. 2013; 111:465–507. PMID: 24267359.
Article6. Bouic PJ. A review of the efficacy and safety of Nasaleze™ in the prevention and management of allergic rhinitis. Open Allergy J. 2008; 1:1–4.7. Diethart B, Emberlin JC, Lewis R. Hydroxypropylmethylcellulose gel application delays Der p 1 diffusion in vitro. Nat Sci (Irvine). 2010; 2:79–84.
Article8. Åberg N, Dahl Å, Benson M. A nasally applied cellulose powder in seasonal allergic rhinitis (SAR) in children and adolescents; reduction of symptoms and relation to pollen load. Pediatr Allergy Immunol. 2011; 22:594–599. PMID: 21645117.
Article9. Emberlin JC, Lewis RA. A double blind, placebo controlled trial of inert cellulose powder for the relief of symptoms of hay fever in adults. Curr Med Res Opin. 2006; 22:275–285. PMID: 16466599.
Article10. Aivazis V, Bourli E, Maratou E, Mavroudi A, Fountzila E, Aivazi D, et al. Study of mucociliary clearance and peak nasal inspiratory flow rate in children with allergic rhinitis before and after therapy with natural cellulose powder. In : Proceedings of the World Allergy Congress 2005; 2005 Jun 26–Jul 1; Munich, Germany. [place unknown]: Nea Pediatrica Chronica;2005.11. Åberg N, Ospanova ST, Nikitin NP, Emberlin J, Dahl Å. A nasally applied cellulose powder in seasonal allergic rhinitis in adults with grass pollen allergy: a double-blind, randomized, placebo-controlled, parallel-group study. Int Arch Allergy Immunol. 2014; 163:313–318. PMID: 24852424.
Article12. Emberlin JC, Lewis RA. A double blind, placebo-controlled cross over trial of cellulose powder by nasal provocation with Der p1 and Der f1. Curr Med Res Opin. 2007; 23:2423–2431. PMID: 17767803.
Article13. Pipkorn U. Budesonide and nasal allergen challenge testing in man. Allergy. 1982; 37:129–134. PMID: 7137521.
Article14. Dolovich J, Moote DW, Mazza JA, Clermont A, PetitClerc C, Danzig M. Efficacy of loratadine versus placebo in the prophylactic treatment of seasonal allergic rhinitis. Ann Allergy. 1994; 73:235–239. PMID: 8092558.15. Calderon MA, Bernstein DI, Blaiss M, Andersen JS, Nolte H. A comparative analysis of symptom and medication scoring methods used in clinical trials of sublingual immunotherapy for seasonal allergic rhinitis. Clin Exp Allergy. 2014; 44:1228–1239. PMID: 24773171.
Article16. Amar SM, Harbeck RJ, Sills M, Silveira LJ, O'Brien H, Nelson HS. Response to sublingual immunotherapy with grass pollen extract: monotherapy versus combination in a multiallergen extract. J Allergy Clin Immunol. 2009; 124:150–156. 156.e1–156.e5. PMID: 19523672.
Article17. Scadding GW, Hansel TT, Durham SR. Nasal provocation testing. In : Adkinson NF, Bochner BS, editors. Middleton's allergy: principle and practice. 8th ed. Philadelphia (PA): Elsevier;2014. p. 652–663.18. Tantilipikorn P, Vichyanond P, Lacroix JS. Nasal provocation test: how to maximize its clinical use? Asian Pac J Allergy Immunol. 2010; 28:225–231. PMID: 21337904.19. Linder A. Symptom scores as measures of the severity of rhinitis. Clin Allergy. 1988; 18:29–37. PMID: 3349590.
Article20. Özgür A, Arslanoğlu S, Etıt D, Demıray U, Önal HK. Comparison of nasal cytology and symptom scores in patients with seasonal allergic rhinitis, before and after treatment. J Laryngol Otol. 2011; 125:1028–1032. PMID: 21791158.
Article21. Howarth PH, Persson CG, Meltzer EO, Jacobson MR, Durham SR, Silkoff PE. Objective monitoring of nasal airway inflammation in rhinitis. J Allergy Clin Immunol. 2005; 115:S414–S441. PMID: 15746881.
Article22. Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. 2015; 351:h4672. PMID: 26341898.
Article23. Sapp M. Research design. In : Sapp M, editor. Basic psychological measurement, research designs, and statistics without math. Springfield (IL): Charles C. Thomas Publisher;2006. p. 74–89.24. Valerieva A, Popov TA, Staevska M, Kralimarkova T, Petkova E, Valerieva E, et al. Effect of micronized cellulose powder on the efficacy of topical oxymetazoline in allergic rhinitis. Allergy Asthma Proc. 2015; 36:e134–e139. PMID: 26133030.
Article25. Popov TA, Valerieva A, Church MK, Staevska M, Kralimarkova T, Petkova E, et al. Real-life study on the effect of micronized cellulose powder as add-on to intranasal as-needed treatment of subjects with pollen allergic rhinitis. J Allergy Clin Immunol. 2016; 137(Suppl):AB402.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Comparison of the Therapeutic Effects of Powder and Aerosolized Budesonide in the Treatment of Perennial Allergic Rhinitis
- Comparison of cetirizine and terfenadine in the treatmebt of perennial rhinitis
- Therapeutic Effects of Fermented Red Ginseng in Allergic Rhinitis: A Randomized, Double-Blind, Placebo-Controlled Study
- Benefits of Nasal Cellulose Powder Application Depend on the Type of Allergen Sensitization in Allergic Rhinitis
- Allergen-Specific Immunotherapy Against Allergic Respiratory Diseases