Pediatr Gastroenterol Hepatol Nutr.
2015 Dec;18(4):238-245. 10.5223/pghn.2015.18.4.238.
Esophageal Bolus Transit in Newborns with Gastroesophageal Reflux Disease Symptoms: A Multichannel Intraluminal Impedance Study
- Affiliations
-
- 1Neonatal Intensive Care Unit, S.Anna-Regina Margherita Children's Hospital, Torino, Italy. francesco.cresi@unito.it
- 2Department of Pediatrics, University of Torino, Torino, Italy.
- 3Neonatal Intensive Care Unit, Maria Vittoria Hospital, Torino, Italy.
- 4Department of Pediatrics, University of Bari Policlinico, Bari, Italy.
- KMID: 2383301
- DOI: http://doi.org/10.5223/pghn.2015.18.4.238
Abstract
- PURPOSE
The aim of this study was to evaluate bolus transit during esophageal swallow (ES) and gastroesophageal reflux (GER) events and to investigate the relationship between the characteristics of ES and GER events in a population of term and preterm newborns with symptoms of gastroesophageal reflux disease (GERD).
METHODS
The study population consisted of term and preterm newborns referred to combined multichannel intraluminal impedance (MII) and pH monitoring for GERD symptoms. The frequency and characteristics of ES and GER events were assessed by two independent investigators. Statistical significance was set at p<0.05.
RESULTS
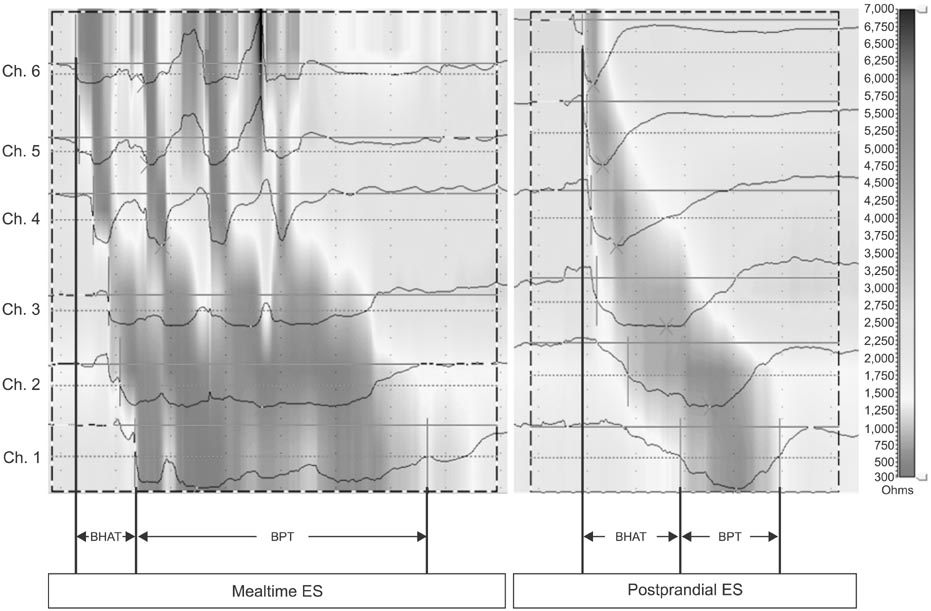
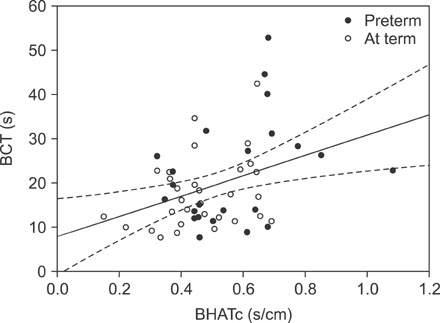
Fifty-four newborns (23 preterm) were included in the analyses. Median bolus head advancing time corrected for esophageal length (BHATc) was shorter during mealtime than during the postprandial period (median, interquartile range): 0.20 (0.15-0.29) s/cm vs. 0.47 (0.39-0.64) s/cm, p<0.001. Median bolus presence time (BPT) was prolonged during mealtime: 4.71(3.49-6.27) s vs. 2.66 (1.82-3.73) s, p<0.001. Higher BHATc (p=0.03) and prolonged BPT (p<0.001) were observed in preterm newborns during the postprandial period. A significant positive correlation between BHATc and bolus clearance time was also observed (rho=0.33, p=0.016).
CONCLUSION
The analysis of ES and GER events at the same time by MII provides useful information to better understand the physiopathology of GERD. In particular, the analysis of BHATc during the postprandial period could help clinicians identify newborns with prolonged esophageal clearance time due to impaired esophageal motility, which could allow for more accurate recommendations regarding further tests and treatment.
MeSH Terms
Figure
Reference
-
1. Vandenplas Y, Hegar B. Diagnosis and treatment of gastro-oesophageal reflux disease in infants and children. J Gastroenterol Hepatol. 2000; 15:593–603.
Article2. Omari TI, Barnett CP, Benninga MA, Lontis R, Goodchild L, Haslam RR, et al. Mechanisms of gastro-oesophageal reflux in preterm and term infants with reflux disease. Gut. 2002; 51:475–479.
Article3. Chitkara DK, Fortunato C, Nurko S. Esophageal motor activity in children with gastro-esophageal reflux disease and esophagitis. J Pediatr Gastroenterol Nutr. 2005; 40:70–75.
Article4. Tutuian R, Vela MF, Shay SS, Castell DO. Multichannel intraluminal impedance in esophageal function testing and gastroesophageal reflux monitoring. J Clin Gastroenterol. 2003; 37:206–215.
Article5. van Wijk MP, Benninga MA, Omari TI. Role of the multichannel intraluminal impedance technique in infants and children. J Pediatr Gastroenterol Nutr. 2009; 48:2–12.
Article6. López-Alonso M, Moya MJ, Cabo JA, Ribas J, del Carmen Macías M, Silny J, et al. Twenty-four-hour esophageal impedance-pH monitoring in healthy preterm neonates: rate and characteristics of acid, weakly acidic, and weakly alkaline gastroesophageal reflux. Pediatrics. 2006; 118:e299–e308.7. Francavilla R, Magistà AM, Bucci N, Villirillo A, Boscarelli G, Mappa L, et al. Comparison of esophageal pH and multichannel intraluminal impedance testing in pediatric patients with suspected gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2010; 50:154–160.
Article8. Corvaglia L, Mariani E, Aceti A, Capretti MG, Ancora G, Faldella G. Combined oesophageal impedance-pH monitoring in preterm newborn: comparison of two options for layout analysis. Neurogastroenterol Motil. 2009; 21:1027–e81.
Article9. Wenzl TG, Skopnik H. Intraluminal impedance: an ideal technique for evaluation of pediatric gastroesophageal reflux disease. Curr Gastroenterol Rep. 2000; 2:259–264.
Article10. Peter CS, Wiechers C, Bohnhorst B, Silny J, Poets CF. Detection of small bolus volumes using multiple intraluminal impedance in preterm infants. J Pediatr Gastroenterol Nutr. 2003; 36:381–384.
Article11. Di Pace MR, Caruso AM, Catalano P, Casuccio A, De Grazia E. Evaluation of esophageal motility using multichannel intraluminal impedance in healthy children and children with gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2011; 52:26–30.
Article12. Imam H, Shay S, Ali A, Baker M. Bolus transit patterns in healthy subjects: a study using simultaneous impedance monitoring, videoesophagram, and esophageal manometry. Am J Physiol Gastrointest Liver Physiol. 2005; 288:G1000–G1006.
Article13. Rudolph CD, Mazur LJ, Liptak GS, Baker RD, Boyle JT, Colletti RB, et al. North American Society for Pediatric Gastroenterology and Nutrition. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2001; 32:Suppl 2. S1–S31.14. Gupta A, Jadcherla SR. The relationship between somatic growth and in vivo esophageal segmental and sphincteric growth in human neonates. J Pediatr Gastroenterol Nutr. 2006; 43:35–41.
Article15. Tutuian R, Castell DO. Clarification of the esophageal function defect in patients with manometric ineffective esophageal motility: studies using combined impedance-manometry. Clin Gastroenterol Hepatol. 2004; 2:230–236.
Article16. Hila A, Chowdhury N, Hajar N, Castell DO. Swallow evaluation during multichannel intraluminal impedance and pH: an alternate method to assess esophageal transit. J Clin Gastroenterol. 2011; 45:862–866.17. Frieling T, Hermann S, Kuhlbusch R, Enck P, Silny J, Lübke HJ, et al. Comparison between intraluminal multiple electric impedance measurement and manometry in the human oesophagus. Neurogastroenterol Motil. 1996; 8:45–50.
Article18. Clayton SB, Rife C, Kalbfleisch JH, Castell DO. Viscous impedance is an important indicator of abnormal esophageal motility. Neurogastroenterol Motil. 2013; 25:563–e455.
Article19. Omari TI, Rommel N, Szczesniak MM, Fuentealba S, Dinning PG, Davidson GP, et al. Assessment of intraluminal impedance for the detection of pharyngeal bolus flow during swallowing in healthy adults. Am J Physiol Gastrointest Liver Physiol. 2006; 290:G183–G188.
Article20. Loots C, Smits M, Omari T, Bennink R, Benninga M, van Wijk M. Effect of lateral positioning on gastroesophageal reflux (GER) and underlying mechanisms in GER disease (GERD) patients and healthy controls. Neurogastroenterol Motil. 2013; 25:222–229.
Article21. van Wijk MP, Benninga MA, Dent J, Lontis R, Goodchild L, McCall LM, et al. Effect of body position changes on postprandial gastroesophageal reflux and gastric emptying in the healthy premature neonate. J Pediatr. 2007; 151:585–590.
Article22. van Wijk MP, Benninga MA, Davidson GP, Haslam R, Omari TI. Small volumes of feed can trigger transient lower esophageal sphincter relaxation and gastroesophageal reflux in the right lateral position in infants. J Pediatr. 2010; 156:744–748.
Article23. Staiano A, Boccia G, Salvia G, Zappulli D, Clouse RE. Development of esophageal peristalsis in preterm and term neonates. Gastroenterology. 2007; 132:1718–1725.
Article24. Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr. 2003; 143:31–38.
Article25. Omari TI, Miki K, Fraser R, Davidson G, Haslam R, Goldsworthy W, et al. Esophageal body and lower esophageal sphincter function in healthy premature infants. Gastroenterology. 1995; 109:1757–1764.
Article26. Gupta A, Gulati P, Kim W, Fernandez S, Shaker R, Jadcherla SR. Effect of postnatal maturation on the mechanisms of esophageal propulsion in preterm human neonates: primary and secondary peristalsis. Am J Gastroenterol. 2009; 104:411–419.
Article27. Lang IM. Brain stem control of the phases of swallowing. Dysphagia. 2009; 24:333–348.
Article28. Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008; 42:610–619.
Article29. Holloway RH. Esophageal body motor response to reflux events: secondary peristalsis. Am J Med. 2000; 108:Suppl 4a. 20S–26S.30. Cresi F, Locatelli E, Marinaccio C, Grasso G, Coscia A, Bertino E. Prognostic values of multichannel intraluminal impedance and pH monitoring in newborns with symptoms of gastroesophageal reflux disease. J Pediatr. 2013; 162:770–775.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Esophageal pH and Combined Impedance-pH Monitoring in Children
- Impaired Esophageal Bolus Transit in Patients with Gastroesophageal Reflux Disease and Abnormal Esophageal Acid Exposure
- Transoral Incisionless Fundoplication Leads to Esophageal Mucosa Healing in Responder Patients Followed up to 2 Years, as Documented by Esophageal Mean Nocturnal Baseline Impedance
- Usefulness of Multichannel Intraluminal Impedance-pH Metry in Children with Suspected Gastroesophageal Reflux Disease
- Esophageal Motor Dysfunctions in Gastroesophageal Reflux Disease and Therapeutic Perspectives