J Pathol Transl Med.
2015 Jan;49(1):5-12. 10.4132/jptm.2014.10.24.
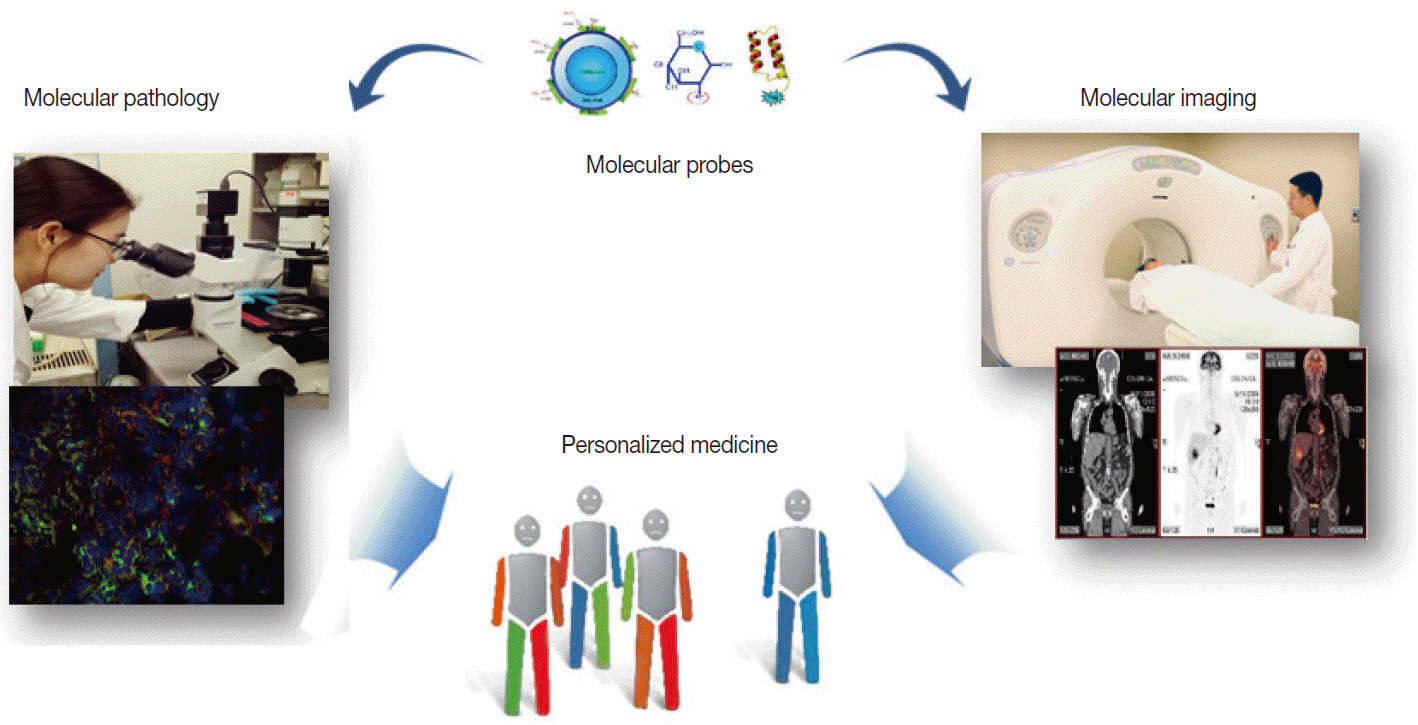
Molecular Imaging in the Era of Personalized Medicine
- Affiliations
-
- 1Department of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. khnm.lee@samsung.com
- KMID: 2381347
- DOI: http://doi.org/10.4132/jptm.2014.10.24
Abstract
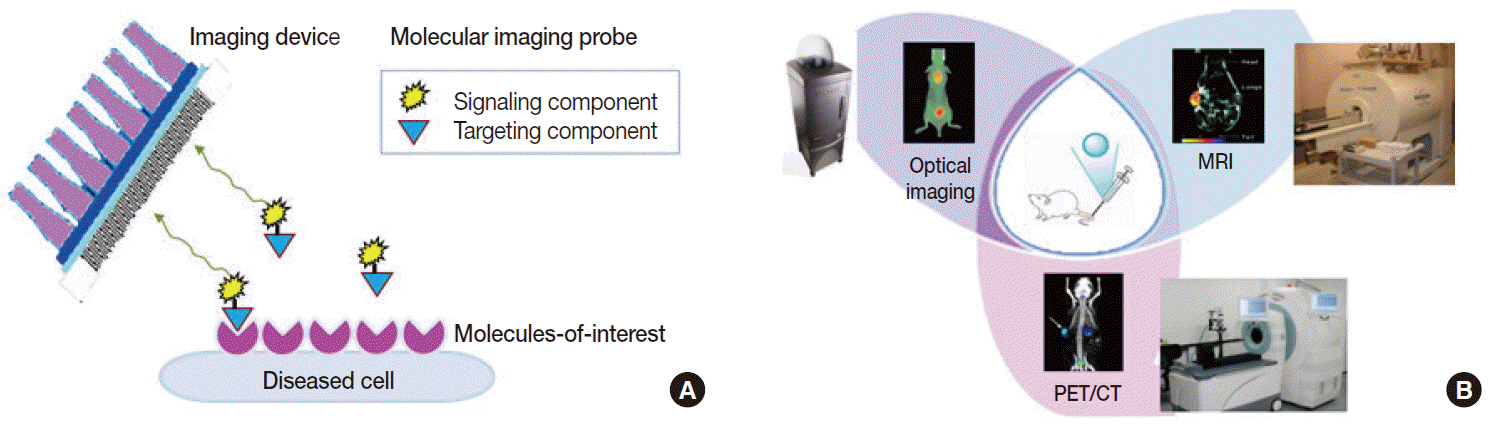
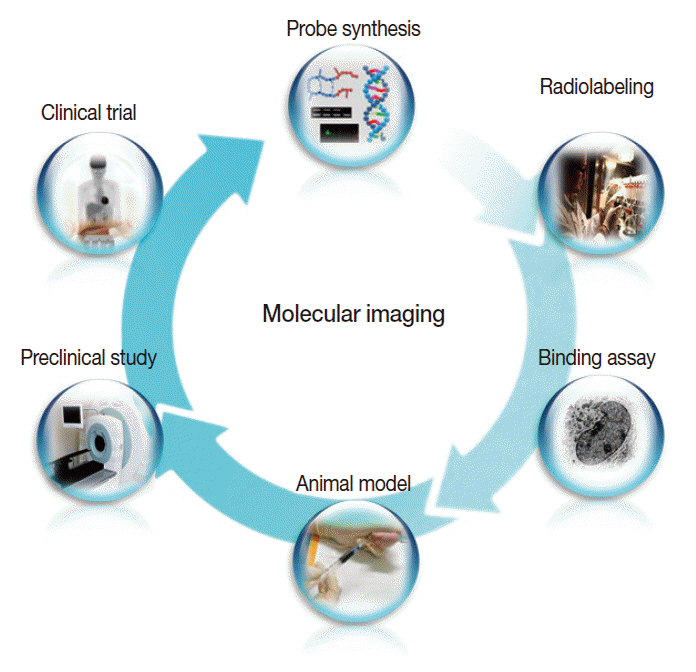
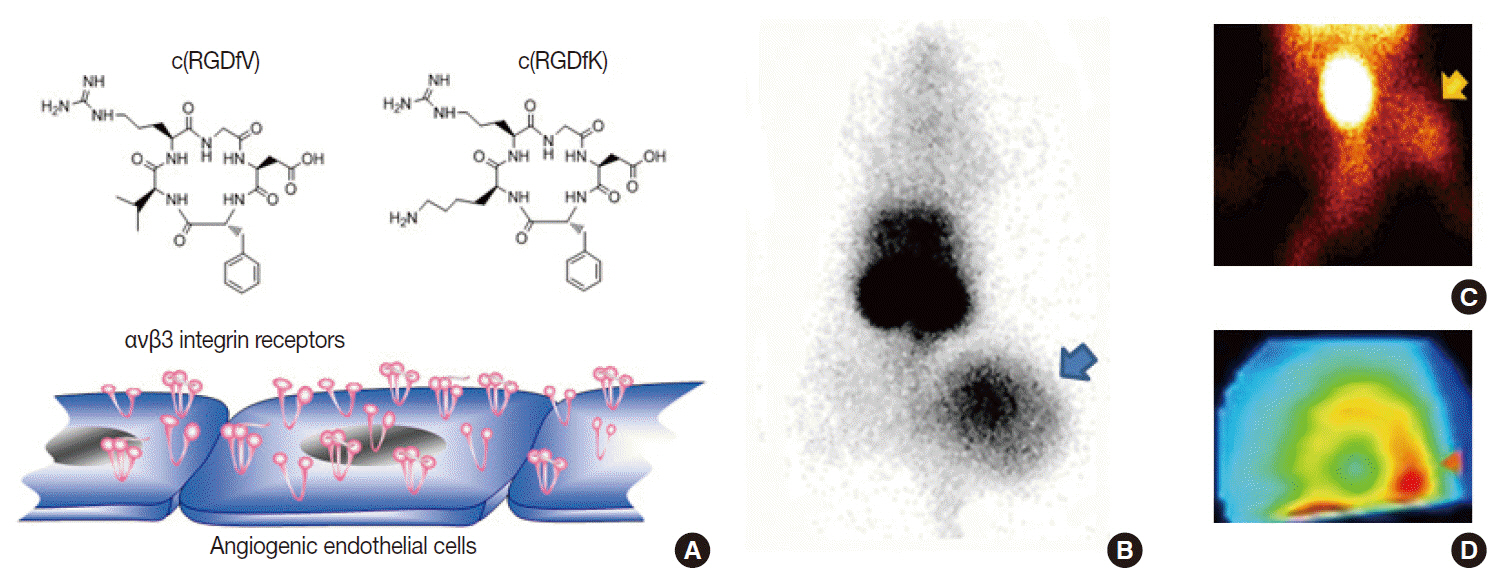
- Clinical imaging creates visual representations of the body interior for disease assessment. The role of clinical imaging significantly overlaps with that of pathology, and diagnostic workflows largely depend on both fields. The field of clinical imaging is presently undergoing a radical change through the emergence of a new field called molecular imaging. This new technology, which lies at the intersection between imaging and molecular biology, enables noninvasive visualization of biochemical processes at the molecular level within living bodies. Molecular imaging differs from traditional anatomical imaging in that biomarkers known as imaging probes are used to visualize target molecules-of-interest. This ability opens up exciting new possibilities for applications in oncologic, neurological and cardiovascular diseases. Molecular imaging is expected to make major contributions to personalized medicine by allowing earlier diagnosis and predicting treatment response. The technique is also making a huge impact on pharmaceutical development by optimizing preclinical and clinical tests for new drug candidates. This review will describe the basic principles of molecular imaging and will briefly touch on three examples (from an immense list of new techniques) that may contribute to personalized medicine: receptor imaging, angiogenesis imaging, and apoptosis imaging.
MeSH Terms
Figure
Reference
-
1. Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010; 363:301–4.
Article2. Hoggatt J. Personalized medicine: trends in molecular diagnostics: exponential growth expected in the next ten years. Mol Diagn Ther. 2011; 15:53–5.3. Herschman HR. Molecular imaging: looking at problems, seeing solutions. Science. 2003; 302:605–8.
Article4. Kircher MF, Hricak H, Larson SM. Molecular imaging for personalized cancer care. Mol Oncol. 2012; 6:182–95.
Article5. Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008; 7:591–607.
Article6. Reynolds F, Kelly KA. Techniques for molecular imaging probe design. Mol Imaging. 2011; 10:407–19.
Article7. Weber WA, Grosu AL, Czernin J. Technology Insight: advances in molecular imaging and an appraisal of PET/CT scanning. Nat Clin Pract Oncol. 2008; 5:160–70.
Article8. Gore JC, Manning HC, Quarles CC, Waddell KW, Yankeelov TE. Magnetic resonance in the era of molecular imaging of cancer. Magn Reson Imaging. 2011; 29:587–600.
Article9. Ritman EL. Molecular imaging in small animals: roles for micro-CT. J Cell Biochem Suppl. 2002; 39:116–24.10. Hellebust A, Richards-Kortum R. Advances in molecular imaging: targeted optical contrast agents for cancer diagnostics. Nanomedicine (Lond). 2012; 7:429–45.
Article11. Mankoff DA, Link JM, Linden HM, Sundararajan L, Krohn KA. Tumor receptor imaging. J Nucl Med. 2008; 49 Suppl 2:149S–63S.
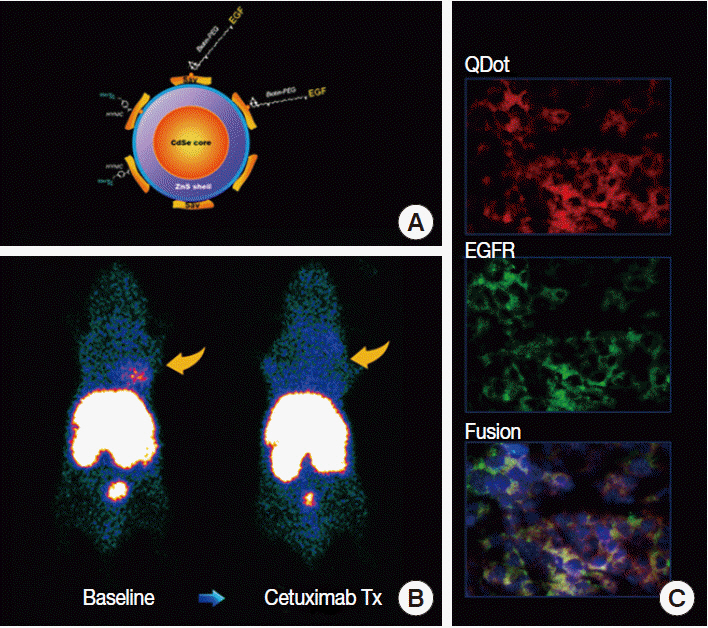
Article12. Smith-Jones PM, Solit DB, Akhurst T, Afroze F, Rosen N, Larson SM. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol. 2004; 22:701–6.
Article13. Jung KH, Choe YS, Paik JY, Lee KH. 99mTc-Hydrazinonicotinamide epidermal growth factor-polyethylene glycol-quantum dot imaging allows quantification of breast cancer epidermal growth factor receptor expression and monitors receptor downregulation in response to cetuximab therapy. J Nucl Med. 2011; 52:1457–64.14. Choe YS, Lee KH. Targeted in vivo imaging of angiogenesis: present status and perspectives. Curr Pharm Des. 2007; 13:17–31.15. Iagaru A, Gambhir SS. Imaging tumor angiogenesis: the road to clinical utility. AJR Am J Roentgenol. 2013; 201:W183–91.
Article16. Jung KH, Lee KH, Paik JY, et al. Favorable biokinetic and tumor-targeting properties of 99mTc-labeled glucosamino RGD and effect of paclitaxel therapy. J Nucl Med. 2006; 47:2000–7.17. Lee KH, Jung KH, Song SH, et al. Radiolabeled RGD uptake and alphav integrin expression is enhanced in ischemic murine hindlimbs. J Nucl Med. 2005; 46:472–8.18. Yang TJ, Haimovitz-Friedman A, Verheij M. Anticancer therapy and apoptosis imaging. Exp Oncol. 2012; 34:269–76.19. Jung KH, Lee JH, Park JW, et al. Annexin V imaging detects diabetes-accelerated apoptosis and monitors the efficacy of benfotiamine treatment in ischemic limbs of mice. Mol Imaging. 2014; 13:1–7.
Article20. Kartachova M, van Zandwijk N, Burgers S, van Tinteren H, Verheij M, Valdés Olmos RA. Prognostic significance of 99mTc Hynic-rhannexin V scintigraphy during platinum-based chemotherapy in advanced lung cancer. J Clin Oncol. 2007; 25:2534–9.
Article21. Allen AM, Ben-Ami M, Reshef A, et al. Assessment of response of brain metastases to radiotherapy by PET imaging of apoptosis with 18F-ML-10. Eur J Nucl Med Mol Imaging. 2012; 39:1400–8.