Cancer Res Treat.
2014 Oct;46(4):419-424. 10.4143/crt.2013.104.
Bladder and Liver Involvement of Visceral Larva Migrans May Mimic Malignancy
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. yhk0215@korea.ac.kr
- KMID: 2380381
- DOI: http://doi.org/10.4143/crt.2013.104
Abstract
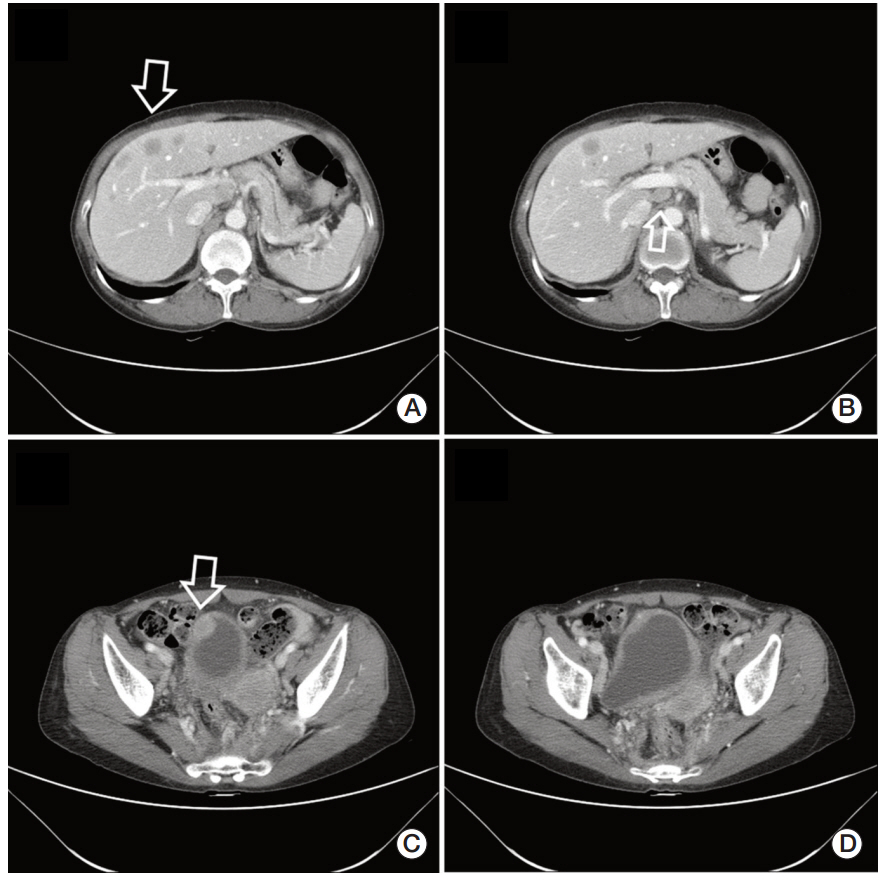
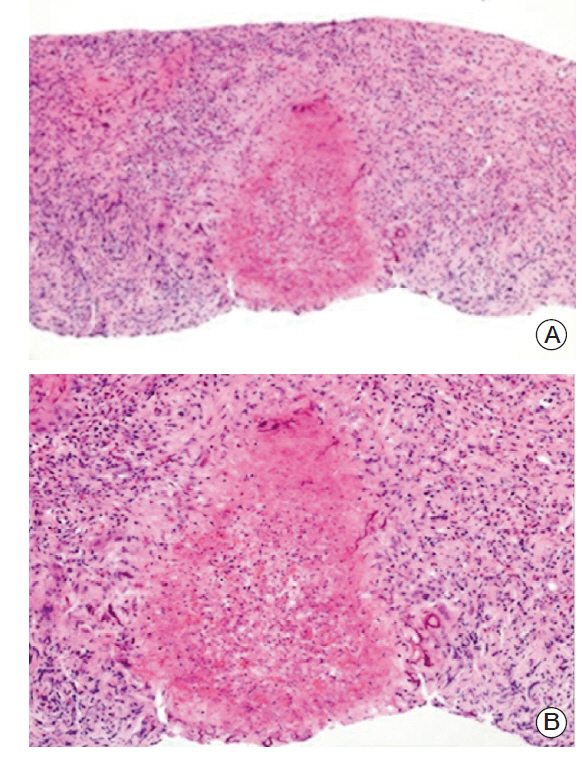
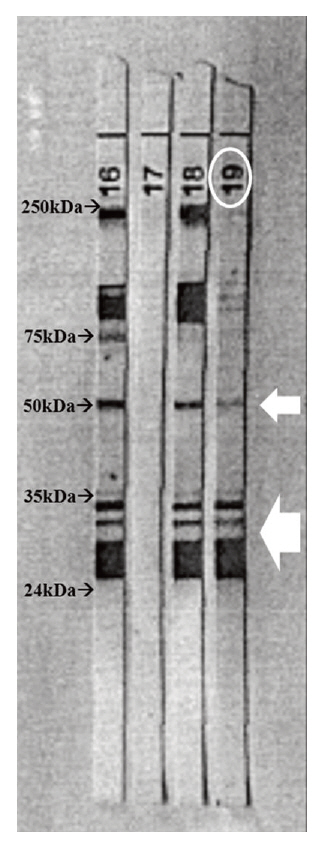
- Visceral larva migrans (VLM) syndrome is a clinical manifestation of systemic organ involvement by Toxocara species. VLM with involvement of the bladder and liver is a rare finding. A 62-year-old woman presented with diffuse bladder wall thickening and multiple liver masses with peripheral eosinophilia and urinary symptoms. We considered malignancy or eosinophilic cystitis through clinical manifestations and imaging findings. However, no suspicious malignant lesions were observed on cystoscopy and liver mass biopsy revealed the presence of eosinophilic necrotizing granuloma without malignant cells. Anti-Toxocara antibodies were detected by western blotting and the patient was diagnosed with VLM syndrome. After taking prednisolone, urinary symptoms disappeared. On abdominal CT scan taken after three months, the size of multiple liver masses and bladder wall thickening had decreased. VLM syndrome should be suspected in patients with an atypical imaging pattern and peripheral eosinophilia.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Beaver PC, Snyder CH, Carrera GM, Dent JH, Lafferty JW. Chronic eosinophilia due to visceral larva migrans; report of three cases. Pediatrics. 1952; 9:7–19.2. Woodhall D, Starr MC, Montgomery SP, Jones JL, Lum F, Read RW, et al. Ocular toxocariasis: epidemiologic, anatomic, and therapeutic variations based on a survey of ophthalmic subspecialists. Ophthalmology. 2012; 119:1211–7.
Article3. Hossack J, Ricketts P, Te HS, Hart J. A case of adult hepatic toxocariasis. Nat Clin Pract Gastroenterol Hepatol. 2008; 5:344–8.
Article4. Lim YJ, Kim JH, Oh SH, Jeon SC, Koh HC, Lee YH. Pulmonary toxocariasis masquerading as metastatic tumor nodules in a child with osteosarcoma. Pediatr Blood Cancer. 2009; 53:1343–5.
Article5. Gutierrez Y. Diagnostic pathology of parasitic infections with clinical correlations. New York: Oxford University Press;2000.6. Dao AH, Virmani R. Visceral larva migrans involving the myocardium: report of two cases and review of literature. Pediatr Pathol. 1986; 6:449–56.
Article7. Singer OC, Conrad F, Jahnke K, Hattingen E, Auer H, Stein-metz H. Severe meningoencephalomyelitis due to CNS-Toxocarosis. J Neurol. 2011; 258:696–8.
Article8. Azuma K, Yashiro N, Kinoshita T, Yoshigi J, Ihara N. Hepatic involvement of visceral larva migrans due to Toxocara canis: a case report: CT and MR findings. Radiat Med. 2002; 20:89–92.9. Wong-You-Cheong JJ, Woodward PJ, Manning MA, Davis CJ. From the archives of the AFIP: Inflammatory and nonneoplastic bladder masses: radiologic-pathologiccorrelation. Radiographics. 2006; 26:1847–68.10. Popert RJ, Ramsay JW, Owen RA, Fisher C, Hendry WF. Eosinophilic cystitis mimicking invasive bladder tumour: discussion paper. J R Soc Med. 1990; 83:776–8.
Article11. Iddawela RD, Rajapakse RP, Perera NA, Agatsuma T. Characterization of a Toxocara canis species-specific excretory-secretory antigen (TcES-57) and development of a double sandwich ELISA for diagnosis of visceral larva migrans. Korean J Parasitol. 2007; 45:19–26.
Article12. Bellanger AP, Humbert P, Gavignet B, Deschaseaux AD, Barisien C, Roussel S, et al. Comparative assessment of enzyme-linked immunosorbent assay and Western blot for the diagnosis of toxocariasis in patients with skin disorders. Br J Dermatol. 2010; 162:80–2.
Article13. Fillaux J, Magnaval JF. Laboratory diagnosis of human toxocariasis. Vet Parasitol. 2013; 193:327–36.
Article14. Magnaval JF, Fabre R, Maurieres P, Charlet JP, de Larrard B. Application of the western blotting procedure for the immun-odiagnosis of human toxocariasis. Parasitol Res. 1991; 77:697–702.
Article15. Othman AA. Therapeutic battle against larval toxocariasis: are we still far behind? Acta Trop. 2012; 124:171–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Toxocariasis with Visceral Larva Migrans Combined with Ocular Larva Migrans
- Toxocariasis: A Rare Cause of Multiple Cerebral Infarction
- Two Cases of Cutaneous Larva Migrans
- Foodborne Eosinophilia due to Visceral Larva Migrans: A Disease Abandoned
- Hepatic Toxocariasis with Atypical CT and MR Imaging Findings: a Case Report