Int J Thyroidol.
2017 May;10(1):24-35. 10.11106/ijt.2017.10.1.24.
The Cancer Genome Atlas Validation of Ancillary Tests for Classifying Papillary Thyroid Carcinoma
- Affiliations
-
- 1Department of Surgery, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea. nicizm@gmail.com
- 2Cancer Research Institute, Myongji Hospital, Goyang, Korea.
- 3Department of Pathology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- 4Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2379358
- DOI: http://doi.org/10.11106/ijt.2017.10.1.24
Abstract
- BACKGROUND AND OBJECTIVES
Ancillary tests such as BRAF(V600E) mutation or immunohistochemical (IHC) assays have been utilized as complements to morphological criteria in diagnosing subsets of papillary thyroid carcinoma (PTC). Utilizing results from analysis by The Cancer Genome Atlas (TCGA), we evaluated the diagnostic value and feasibility of these ancillary tests in diagnosing follicular variant PTC (FVPTC).
MATERIALS AND METHODS
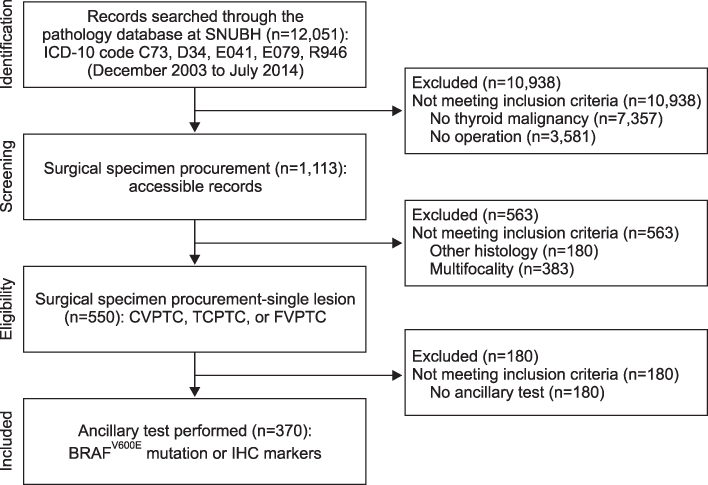
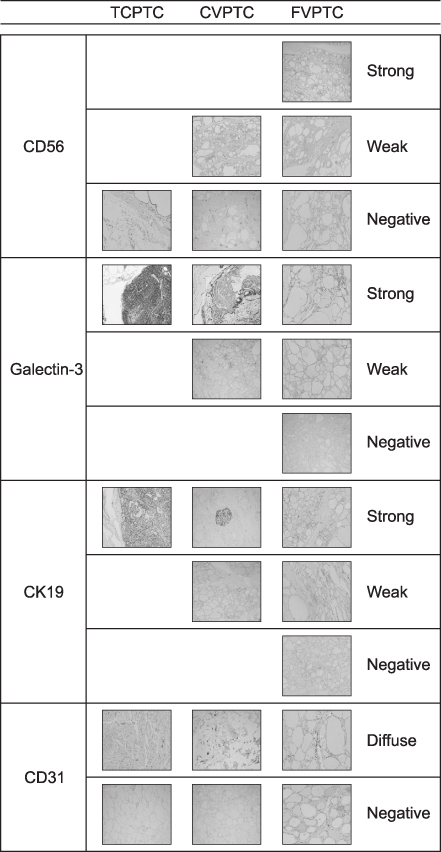
Clinical data and tissue samples were analyzed from 370 PTC patients, who had undergone thyroidectomy between December 2003 and July 2014. PTC was limited to conventional PTC (CVPTC), tall cell variant PTC (TCPTC), and FVPTC. Using multivariate analyses, FVPTC cases were compared to CVPTC and TCPTC cases. Surgical specimens were pyrosequenced for BRAF(V600E) mutation or stained for IHC markers such as CD56, galectin-3, cytokeratin 19 (CK19), or CD31. For the validation, a comprehensive analysis was performed for BRAF(V600E) mutation and quantitative mRNA expressional difference in TCGA.
RESULTS
Demographic differences were not observed between 159 CVPTC, 103 TCPTC, and 108 FVPTC cases. BRAF(V600E) mutation predominated in CVPTC+TCPTC group, but not in FVPTC group (78.4% vs. 18.7%, p<0.001), as suggested by TCGA (57.4% vs. 12.1%, p<0.0001). IHC markers significantly distinguished FVPTC cases from CVPTC+TCPTC cases. CD56 exhibited more intense staining in FVPTC cases (21.1% vs. 72.0%, p<0.001). Galectin-3 and CK19 yielded limited values. CD31 correlated with lymphovascular invasion (r=0.847, p<0.001). In analysis of TCGA, mRNA differential expression of these genes revealed their corresponding upregulation or downregulation.
CONCLUSION
BRAF(V600E) mutation or TCGA-validated IHC assay could be recommended as ancillary tests for classifying PTC.
Keyword
MeSH Terms
Figure
Reference
-
1. Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer "epidemic"--screening and overdiagnosis. N Engl J Med. 2014; 371(19):1765–1767.
Article2. LiVolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011; 24:Suppl 2. S1–S9.
Article3. Schneider DF, Chen H. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin. 2013; 63(6):374–394.
Article4. Salajegheh A, Petcu EB, Smith RA, Lam AK. Follicular variant of papillary thyroid carcinoma: a diagnostic challenge for clinicians and pathologists. Postgrad Med J. 2008; 84(988):78–82.
Article5. Chetty R. Follicular patterned lesions of the thyroid gland: a practical algorithmic approach. J Clin Pathol. 2011; 64(9):737–741.
Article6. Elsheikh TM, Asa SL, Chan JK, DeLellis RA, Heffess CS, LiVolsi VA, et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol. 2008; 130(5):736–744.
Article7. Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004; 28(10):1336–1340.
Article8. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013; 381(9871):1058–1069.
Article9. Ritterhouse LL, Barletta JA. BRAF V600E mutation-specific antibody: A review. Semin Diagn Pathol. 2015; 32(5):400–408.
Article10. Cheng S, Serra S, Mercado M, Ezzat S, Asa SL. A highthroughput proteomic approach provides distinct signatures for thyroid cancer behavior. Clin Cancer Res. 2011; 17(8):2385–2394.
Article11. Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006; 30(2):216–222.
Article12. Capper D, Preusser M, Habel A, Sahm F, Ackermann U, Schindler G, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011; 122(1):11–19.
Article13. Wiseman SM, Melck A, Masoudi H, Ghaidi F, Goldstein L, Gown A, et al. Molecular phenotyping of thyroid tumors identifies a marker panel for differentiated thyroid cancer diagnosis. Ann Surg Oncol. 2008; 15(10):2811–2826.
Article14. Zhao M, Wang KJ, Tan Z, Zheng CM, Liang Z, Zhao JQ. Identification of potential therapeutic targets for papillary thyroid carcinoma by bioinformatics analysis. Oncol Lett. 2016; 11(1):51–58.
Article15. Serra S, Asa SL. Controversies in thyroid pathology: the diagnosis of follicular neoplasms. Endocr Pathol. 2008; 19(3):156–165.
Article16. Fadda G, Rossi ED. Immunohistochemical diagnosis of thyroid tumors. Surg Pathol Clin. 2014; 7(4):491–500.
Article17. El Demellawy D, Nasr A, Alowami S. Application of CD56, P63 and CK19 immunohistochemistry in the diagnosis of papillary carcinoma of the thyroid. Diagn Pathol. 2008; 3:5.
Article18. Nechifor-Boila A, Borda A, Sassolas G, Hafdi-Nejjari Z, Borson-Chazot F, Lifante JC, et al. Immunohistochemical markers in the diagnosis of papillary thyroid carcinomas: The promising role of combined immunostaining using HBME-1 and CD56. Pathol Res Pract. 2013; 209(9):585–592.
Article19. Dunderovic D, Lipkovski JM, Boricic I, Soldatovic I, Bozic V, Cvejic D, et al. Defining the value of CD56, CK19, Galectin 3 and HBME-1 in diagnosis of follicular cell derived lesions of thyroid with systematic review of literature. Diagn Pathol. 2015; 10:196.
Article20. Alshenawy HA. Utility of immunohistochemical markers in diagnosis of follicular cell derived thyroid lesions. Pathol Oncol Res. 2014; 20(4):819–828.
Article21. Saleh HA, Jin B, Barnwell J, Alzohaili O. Utility of immunohistochemical markers in differentiating benign from malignant follicular-derived thyroid nodules. Diagn Pathol. 2010; 5:9.
Article22. Barut F, Onak Kandemir N, Bektas S, Bahadir B, Keser S, Ozdamar SO. Universal markers of thyroid malignancies: galectin-3, HBME-1, and cytokeratin-19. Endocr Pathol. 2010; 21(2):80–89.
Article23. Sethi K, Sarkar S, Das S, Mohanty B, Mandal M. Biomarkers for the diagnosis of thyroid cancer. J Exp Ther Oncol. 2010; 8(4):341–352.24. Isic Dencic T, Cvejic D, Paunovic I, Tatic S, Havelka M, Savin S. Cytokeratin19 expression discriminates papillary thyroid carcinoma from other thyroid lesions and predicts its aggressive behavior. Med Oncol. 2013; 30(1):362.
Article25. Lee SH, Lee SJ, Jin SM, Lee NH, Kim DH, Chae SW, et al. Relationships between lymph node metastasis and expression of CD31, D2-40, and vascular endothelial growth factors A and C in papillary thyroid cancer. Clin Exp Otorhinolaryngol. 2012; 5(3):150–155.
Article26. Asa SL, Giordano TJ, LiVolsi VA. Implications of the TCGA genomic characterization of papillary thyroid carcinoma for thyroid pathology: does follicular variant papillary thyroid carcinoma exist? Thyroid. 2015; 25(1):1–2.
Article27. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159(3):676–690.28. Giordano TJ. Follicular cell thyroid neoplasia: insights from genomics and The Cancer Genome Atlas research network. Curr Opin Oncol. 2016; 28(1):1–4.29. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016; 2(8):1023–1029.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concurrent Medullay and Papillary Carcinoma of the Thyroid
- A Case of Ectopic Thyroid Papillary Carcinoma with Incidental Papillary Thyroid Microcarcinoma
- Genomic Characterization of Differentiated Thyroid Carcinoma
- Concurrent Papillary and Medullary Carcinoma of the Thyroid Gland
- Occult papillary carcinoma of the thyroid