Genomic Characterization of Differentiated Thyroid Carcinoma
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. yjparkmd@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2441673
- DOI: http://doi.org/10.3803/EnM.2019.34.1.1
Abstract
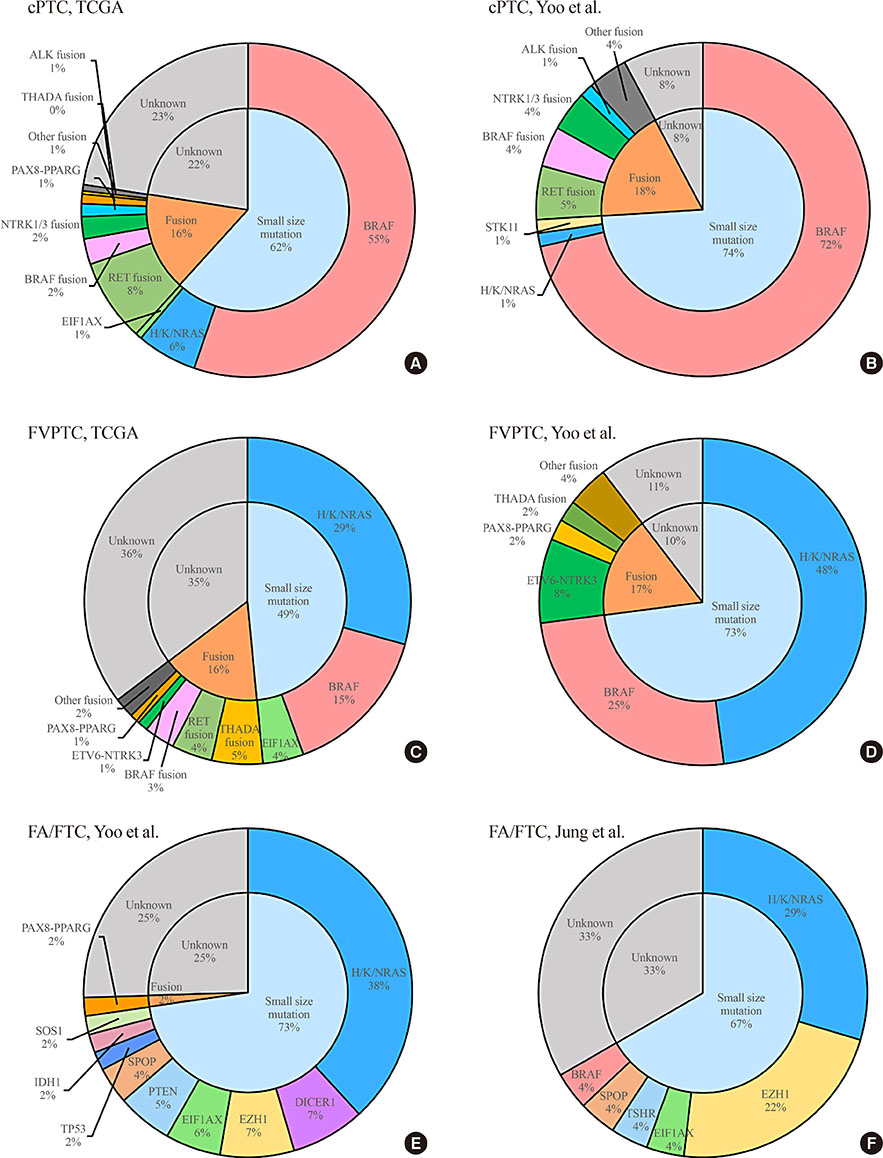
- Since the release of The Cancer Genome Atlas study of papillary thyroid carcinoma (PTC) in 2014, additional genomic studies of differentiated thyroid carcinoma (DTC) using massively-parallel sequencing (MPS) have been published. Recent advances in MPS technology have started to provide important insights into the molecular pathogenesis of DTC. In the genomic landscape, the most recurrently altered genes in DTC, which has a low mutational burden relative to other cancers, are BRAF, RAS, and fusion genes. Some novel driver candidates also have been identified. The frequency of these genomic alterations varies across the subtypes of DTC (classical PTC, follicular variant of PTC, and follicular thyroid carcinoma). Telomerase reverse transcriptase (TERT) promoter mutations are the alteration that makes the most important contribution to the progression of DTC. In the transcriptomic landscape, DTC can be classified according to its gene expression profile, and each subtype has a distinct mutational profile, intracellular signaling output, and clinicopathological characteristics. Herein, we review the results of genomic studies using MPS technology, and describe the types and frequencies of genomic alterations according to histological classifications of DTC and the characteristics and significance of the gene expression signatures of DTC.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Recent Improvements in Genomic and Transcriptomic Understanding of Anaplastic and Poorly Differentiated Thyroid Cancers
Seong-Keun Yoo, Young Shin Song, Young Joo Park, Jeong-Sun Seo
Endocrinol Metab. 2020;35(1):44-54. doi: 10.3803/EnM.2020.35.1.44.Mechanisms of
TERT Reactivation and Its Interaction withBRAF V600E
Young Shin Song, Young Joo Park
Endocrinol Metab. 2020;35(3):515-525. doi: 10.3803/EnM.2020.304.Association of Hyperparathyroidism and Papillary Thyroid Cancer: A Multicenter Retrospective Study (
Endocrinol Metab 2020;35:925–32, Chaiho Jeong et al.)
Chaiho Jeong, Jeonghoon Ha, Moo Il Kang
Endocrinol Metab. 2021;36(1):205-206. doi: 10.3803/EnM.2021.100.Comparative Analysis of Driver Mutations and Transcriptomes in Papillary Thyroid Cancer by Region of Residence in South Korea
Jandee Lee, Seonhyang Jeong, Hwa Young Lee, Sunmi Park, Meesson Jeong, Young Suk Jo
Endocrinol Metab. 2023;38(6):720-728. doi: 10.3803/EnM.2023.1758.
Reference
-
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Martincorena I, Campbell PJ. Somatic mutation in cancer and normal cells. Science. 2015; 349:1483–1489.
Article3. Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017; 545:175–180.4. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–690.5. van Dijk EL, Auger H, Jaszczyszyn Y, Thermes C. Ten years of next-generation sequencing technology. Trends Genet. 2014; 30:418–426.
Article6. Yoo SK, Lee S, Kim SJ, Jee HG, Kim BA, Cho H, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016; 12:e1006239.
Article7. Dralle H, Machens A, Basa J, Fatourechi V, Franceschi S, Hay ID, et al. Follicular cell-derived thyroid cancer. Nat Rev Dis Primers. 2015; 1:15077.
Article8. Gianoukakis AG, Giannelli SM, Salameh WA, McPhaul LW. Well differentiated follicular thyroid neoplasia: impact of molecular and technological advances on detection, monitoring and treatment. Mol Cell Endocrinol. 2011; 332:9–20.
Article9. Mitsutake N, Miyagishi M, Mitsutake S, Akeno N, Mesa C Jr, Knauf JA, et al. BRAF mediates RET/PTC-induced mitogen-activated protein kinase activation in thyroid cells: functional support for requirement of the RET/PTC-RAS-BRAF pathway in papillary thyroid carcinogenesis. Endocrinology. 2006; 147:1014–1019.
Article10. Tallini G, Asa SL. RET oncogene activation in papillary thyroid carcinoma. Adv Anat Pathol. 2001; 8:345–354.
Article11. Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005; 65:4238–4245.
Article12. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017; 49:292–305.
Article13. Martin-Marcos P, Zhou F, Karunasiri C, Zhang F, Dong J, Nanda J, et al. eIF1A residues implicated in cancer stabilize translation preinitiation complexes and favor suboptimal initiation sites in yeast. Elife. 2017; 6:e31250.
Article14. Robertson AG, Shih J, Yau C, Gibb EA, Oba J, Mungall KL, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell. 2017; 32:204–220.
Article15. Martin M, Masshofer L, Temming P, Rahmann S, Metz C, Bornfeld N, et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat Genet. 2013; 45:933–936.
Article16. Liang J, Cai W, Feng D, Teng H, Mao F, Jiang Y, et al. Genetic landscape of papillary thyroid carcinoma in the Chinese population. J Pathol. 2018; 244:215–226.
Article17. Costa V, Esposito R, Ziviello C, Sepe R, Bim LV, Cacciola NA, et al. New somatic mutations and WNK1-B4GALNT3 gene fusion in papillary thyroid carcinoma. Oncotarget. 2015; 6:11242–11251.18. Pan W, Zhou L, Ge M, Zhang B, Yang X, Xiong X, et al. Whole exome sequencing identifies lncRNA GAS8-AS1 and LPAR4 as novel papillary thyroid carcinoma driver alternations. Hum Mol Genet. 2016; 25:1875–1884.19. Lu Z, Zhang Y, Feng D, Sheng J, Yang W, Liu B. Targeted next generation sequencing identifies somatic mutations and gene fusions in papillary thyroid carcinoma. Oncotarget. 2017; 8:45784–45792.
Article20. Siraj AK, Masoodi T, Bu R, Beg S, Al-Sobhi SS, Al-Dayel F, et al. Genomic profiling of thyroid cancer reveals a role for thyroglobulin in metastasis. Am J Hum Genet. 2016; 98:1170–1180.
Article21. Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for follicular thyroid carcinoma: application to 171 consecutive patients treated in a tertiary referral centre. Endocr Relat Cancer. 2007; 14:29–42.
Article22. McHenry CR, Phitayakorn R. Follicular adenoma and carcinoma of the thyroid gland. Oncologist. 2011; 16:585–593.
Article23. Karunamurthy A, Panebianco F, J Hsiao S, Vorhauer J, Nikiforova MN, Chiosea S, et al. Prevalence and phenotypic correlations of EIF1AX mutations in thyroid nodules. Endocr Relat Cancer. 2016; 23:295–301.
Article24. Foulkes WD, Priest JR, Duchaine TF. DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer. 2014; 14:662–672.
Article25. Rutter MM, Jha P, Schultz KA, Sheil A, Harris AK, Bauer AJ, et al. DICER1 mutations and differentiated thyroid carcinoma: evidence of a direct association. J Clin Endocrinol Metab. 2016; 101:1–5.26. de Kock L, Sabbaghian N, Soglio DB, Guillerman RP, Park BK, Chami R, et al. Exploring the association between DICER1 mutations and differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2014; 99:E1072–E1077.27. Rio Frio T, Bahubeshi A, Kanellopoulou C, Hamel N, Niedziela M, Sabbaghian N, et al. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA. 2011; 305:68–77.28. Schultz KA, Pacheco MC, Yang J, Williams GM, Messinger Y, Hill DA, et al. Ovarian sex cord-stromal tumors, pleuropulmonary blastoma and DICER1 mutations: a report from the International Pleuropulmonary Blastoma Registry. Gynecol Oncol. 2011; 122:246–250.
Article29. Calebiro D, Grassi ES, Eszlinger M, Ronchi CL, Godbole A, Bathon K, et al. Recurrent EZH1 mutations are a second hit in autonomous thyroid adenomas. J Clin Invest. 2016; 126:3383–3388.
Article30. Beltran H, Yelensky R, Frampton GM, Park K, Downing SR, MacDonald TY, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol. 2013; 63:920–926.
Article31. Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell. 2015; 163:1011–1025.32. Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012; 44:685–689.
Article33. Hollenbach AD, McPherson CJ, Mientjes EJ, Iyengar R, Grosveld G. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J Cell Sci. 2002; 115(Pt 16):3319–3330.
Article34. Ye L, Zhou X, Huang F, Wang W, Qi Y, Xu H, et al. The genetic landscape of benign thyroid nodules revealed by whole exome and transcriptome sequencing. Nat Commun. 2017; 8:15533.
Article35. Jung SH, Kim MS, Jung CK, Park HC, Kim SY, Liu J, et al. Mutational burdens and evolutionary ages of thyroid follicular adenoma are comparable to those of follicular carcinoma. Oncotarget. 2016; 7:69638–69648.
Article36. Yang H, Ye D, Guan KL, Xiong Y. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin Cancer Res. 2012; 18:5562–5571.
Article37. Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol. 2016; 34:155–163.
Article38. Rosai J, Carcangiu ML, DeLellis RA. Tumors of the thyroid gland. Washington DC: Armed Forces Institute of Pathology;1992.39. Giordano TJ. Follicular cell thyroid neoplasia: insights from genomics and The Cancer Genome Atlas research network. Curr Opin Oncol. 2016; 28:1–4.40. Lam AK, Lo CY, Lam KS. Papillary carcinoma of thyroid: a 30-yr clinicopathological review of the histological variants. Endocr Pathol. 2005; 16:323–330.
Article41. Hofree M, Shen JP, Carter H, Gross A, Ideker T. Network-based stratification of tumor mutations. Nat Methods. 2013; 10:1108–1115.
Article42. Wreesmann VB, Ghossein RA, Hezel M, Banerjee D, Shaha AR, Tuttle RM, et al. Follicular variant of papillary thyroid carcinoma: genome-wide appraisal of a controversial entity. Genes Chromosomes Cancer. 2004; 40:355–364.
Article43. Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016; 2:1023–1029.44. Song YS, Won JK, Yoo SK, Jung KC, Kim MJ, Kim SJ, et al. Comprehensive transcriptomic and genomic profiling of subtypes of follicular variant of papillary thyroid carcinoma. Thyroid. 2018; 28:1468–1478.
Article45. Rivera M, Ricarte-Filho J, Patel S, Tuttle M, Shaha A, Shah JP, et al. Encapsulated thyroid tumors of follicular cell origin with high grade features (high mitotic rate/tumor necrosis): a clinicopathologic and molecular study. Hum Pathol. 2010; 41:172–180.
Article46. Cheong C, Hong KU, Lee HW. Mouse models for telomere and telomerase biology. Exp Mol Med. 2003; 35:141–153.
Article47. Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005; 6:611–622.
Article48. Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013; 339:959–961.
Article49. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013; 339:957–959.
Article50. Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer. 2016; 23:R143–R155.
Article51. Song YS, Lim JA, Park YJ. Mutation profile of well-differentiated thyroid cancer in Asians. Endocrinol Metab (Seoul). 2015; 30:252–262.
Article52. Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013; 20:603–610.
Article53. Yin DT, Yu K, Lu RQ, Li X, Xu J, Lei M, et al. Clinicopathological significance of TERT promoter mutation in papillary thyroid carcinomas: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2016; 85:299–305.
Article54. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014; 32:2718–2726.
Article55. Song YS, Lim JA, Choi H, Won JK, Moon JH, Cho SW, et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer. 2016; 122:1370–1379.56. Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M. Mortality risk stratification by combining BRAF V600E and TERT promoter mutations in papillary thyroid cancer: genetic duet of BRAF and TERT promoter mutations in thyroid cancer mortality. JAMA Oncol. 2017; 3:202–208.57. Moon S, Song YS, Kim YA, Lim JA, Cho SW, Moon JH, et al. Effects of coexistent BRAF(V600E) and TERT promoter mutations on poor clinical outcomes in papillary thyroid cancer: a meta-analysis. Thyroid. 2017; 27:651–660.58. Song YS, Lim JA, Min HS, Kim MJ, Choi HS, Cho SW, et al. Changes in the clinicopathological characteristics and genetic alterations of follicular thyroid cancer. Eur J Endocrinol. 2017; 177:465–473.
Article59. Cho AB, Yoo SK, Sohn MH, Shin JY, Kim S, Lee EK, et al. Abstract 3398: The genomic and transcriptomic analysis of nine widely invasive follicular thyroid carcinomas (wiFTC) in Korean patients. Cancer Research. 2017; 77:13 Suppl. 3398.
Article60. Nikiforov YE, Biddinger PW, Thompson LDR. Diagnostic pathology and molecular genetics of the thyroid. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins;2012.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Improvements in Genomic and Transcriptomic Understanding of Anaplastic and Poorly Differentiated Thyroid Cancers
- Molecular Pathogenesis and Targeted Therapies in Well-Differentiated Thyroid Carcinoma
- Two cases of thyroid small cell carcinoma
- Metastasis of Poorly Differentiated Thyroid Carcinoma to the Sternum: A Case Report
- Analysis of the mitochondrial D-loop sequence in a patient with thyroid Hurthle cell carcinoma and sacral bone metastasis