Ann Dermatol.
2017 Jun;29(3):295-301. 10.5021/ad.2017.29.3.295.
The Effect of Rhus verniciflua Stokes Extracts on Photo-Aged Mouse Skin
- Affiliations
-
- 1Depatment of Dermatology, Yonsei University Wonju College of Medicine, Wonju, Korea. choieh@yonsei.ac.kr
- 2Department of Food and Nutrition, Sangji Youngseo College, Wonju, Korea.
- KMID: 2378528
- DOI: http://doi.org/10.5021/ad.2017.29.3.295
Abstract
- BACKGROUND
Rhus verniciflua Stokes (RV) has traditionally been used in Korea as an indigenous food (Rhus chicken soup) and as an herbal medicinal plant. While the anticancer, antimicrobial, and anti-inflammatory properties of RV have been actively studied in the medical field, its antioxidant effects in the skin that resist the reactive oxygen species in keratinocytes and fibroblasts is less understood.
OBJECTIVE
We designed to evaluate the effects of R. verniciflua Stokes extract (RVE) on the photo-aged skin by an in vitro experiment using human fibroblasts and an in vivo experiment using a photo-aged murine model.
METHODS
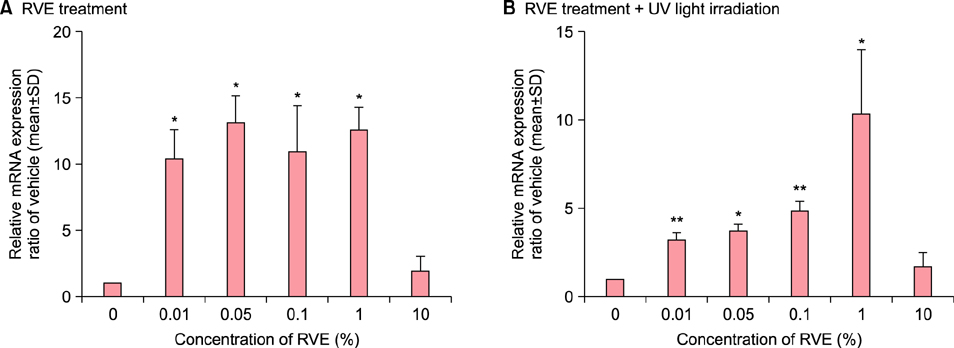
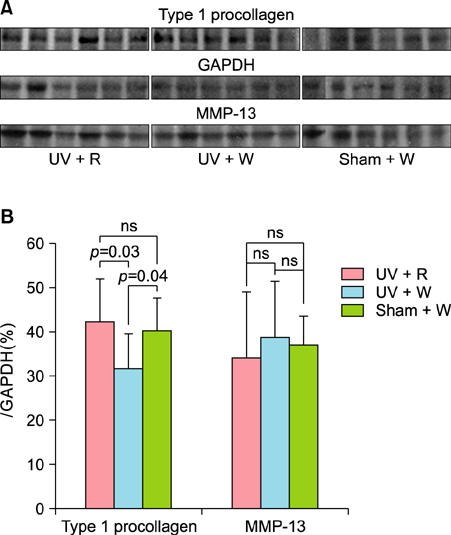
For the in vitro experiments, human fibroblasts irradiated with ultraviolet (UV) B were treated with RVE or vehicle, and the growth levels and the expression level of type 1 procollagen were compared. For the in vivo experiment, photo-aged mice irradiated with UVB and UVA were administered drinking water with or without RVE, and histological changes and the expression level of type 1 procollagen and matrix metalloprotease (MMP)-13 were compared.
RESULTS
In vitro experiments using fibroblasts irradiated with UVB showed that RVE promoted growth and significantly increased the expression of type 1 procollagen as compared to the control group. In the photo-aged mice, RVE increased collagen content in the dermis and promoted the synthesis of type 1 procollagen without any visible decrease in MMP-13 as compared to control group.
CONCLUSION
In addition to the previously reported antioxidant effects of RVE, oral intake of RVE effectively inhibited photo-aging in hairless mice by enhancing collagen synthesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002; 138:1462–1470.
Article2. Chung JH, Hanft VN, Kang S. Aging and photoaging. J Am Acad Dermatol. 2003; 49:690–697.
Article3. Kim JH, Shin YC, Ko SG. Integrating traditional medicine into modern inflammatory diseases care: multitargeting by Rhus verniciflua Stokes. Mediators Inflamm. 2014; 2014:154561.4. Kitts DD, Lim KT. Antitumorigenic and cytotoxic properties of an ethanol extract derived from Rhus verniciflua Stokes (RVS). J Toxicol Environ Health A. 2001; 64:357–371.
Article5. Lim KT, Hu C, Kitts DD. Antioxidant activity of a Rhus verniciflua Stokes ethanol extract. Food Chem Toxicol. 2001; 39:229–237.
Article6. Suk KT, Baik SK, Kim HS, Park SM, Paeng KJ, Uh Y, et al. Antibacterial effects of the urushiol component in the sap of the lacquer tree (Rhus verniciflua Stokes) on Helicobacter pylori. Helicobacter. 2011; 16:434–443.
Article7. Chen H, Wang C, Ye J, Zhou H, Yuan J. Phenolic extracts from Rhus verniciflua Stokes bark by decompressing inner ebullition and their antioxidant activities. Nat Prod Res. 2014; 28:496–499.
Article8. Liu CS, Nam TG, Han MW, Ahn SM, Choi HS, Kim TY, et al. Protective effect of detoxified Rhus verniciflua stokes on human keratinocytes and dermal fibroblasts against oxidative stress and identification of the bioactive phenolics. Biosci Biotechnol Biochem. 2013; 77:1682–1688.
Article9. Kim JB. Method for detoxification of the extracts from Rhus verniciflua. Korea patent;1999.10. Kim IW, Shin DH, Baek NI. Identification of antioxidative components from ethanol extract of Rhus verniciflua Stokes. Korea J Food Sci Technol. 1999; 31:1654–1660.11. Kim JB. Identification of antioxidative component from stem bark of Rhus verniciflua. Korea J Food Nutr. 2003; 16:60–65.12. Park HY, Youm JK, Kwon MJ, Park BD, Lee SH, Choi EH. K6PC-5, a novel sphingosine kinase activator, improves long-term ultraviolet light-exposed aged murine skin. Exp Dermatol. 2008; 17:829–836.
Article13. Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006; 55:1–19.
Article14. Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002; 1:705–720.
Article15. Tanaka H, Yamaba H, Kosugi N, Mizutani H, Nakata S. Fermentable metabolite of Zymomonas mobilis controls collagen reduction in photoaging skin by improving TGF-beta/Smad signaling suppression. Arch Dermatol Res. 2008; 300:Suppl 1. S57–S64.16. Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008; 7:2 Suppl. s12–s16.17. Iannacone MR, Hughes MC, Green AC. Effects of sunscreen on skin cancer and photoaging. Photodermatol Photoimmunol Photomed. 2014; 30:55–61.
Article18. Alexiades-Armenakas M, Sarnoff D, Gotkin R, Sadick N. Multi-center clinical study and review of fractional ablative CO2 laser resurfacing for the treatment of rhytides, photoaging, scars and striae. J Drugs Dermatol. 2011; 10:352–362.19. Dierickx CC, Anderson RR. Visible light treatment of photoaging. Dermatol Ther. 2005; 18:191–208.
Article20. Griffiths CE, Goldfarb MT, Finkel LJ, Roulia V, Bonawitz M, Hamilton TA, et al. Topical tretinoin (retinoic acid) treatment of hyperpigmented lesions associated with photoaging in Chinese and Japanese patients: a vehicle-controlled trial. J Am Acad Dermatol. 1994; 30:76–84.
Article21. Stern RS. Clinical practice. Treatment of photoaging. N Engl J Med. 2004; 350:1526–1534.22. Van Scott EJ, Ditre CM, Yu RJ. Alpha-hydroxyacids in the treatment of signs of photoaging. Clin Dermatol. 1996; 14:217–226.
Article23. Roh SS, Lee MH, Hwang YL, Song HH, Jin MH, Park SG, et al. Stimulation of the extracellular matrix production in dermal fibroblasts by velvet antler extract. Ann Dermatol. 2010; 22:173–179.
Article24. Park HM, Hwang E, Lee KG, Han SM, Cho Y, Kim SY. Royal jelly protects against ultraviolet B-induced photoaging in human skin fibroblasts via enhancing collagen production. J Med Food. 2011; 14:899–906.
Article25. Chiang HM, Chen HC, Lin TJ, Shih IC, Wen KC. Michelia alba extract attenuates UVB-induced expression of matrix metalloproteinases via MAP kinase pathway in human dermal fibroblasts. Food Chem Toxicol. 2012; 50:4260–4269.
Article26. Furumura M, Sato N, Kusaba N, Takagaki K, Nakayama J. Oral administration of French maritime pine bark extract (Flavangenol(®)) improves clinical symptoms in photoaged facial skin. Clin Interv Aging. 2012; 7:275–286.27. OyetakinWhite P, Tribout H, Baron E. Protective mechanisms of green tea polyphenols in skin. Oxid Med Cell Longev. 2012; 2012:560682.
Article28. Lee J, Chae J, Lee S, Kim K, Eo W, Kim S, et al. The efficacy and safety of standardized allergen-removed Rhus verniciflua extract as maintenance therapy after first-line chemotherapy in patients with advanced non-small cell lung cancer. Am J Chin Med. 2013; 41:773–787.
Article29. Lee SK, Jung HS, Eo WK, Lee SY, Kim SH, Shim BS. Rhus verniciflua Stokes extract as a potential option for treatment of metastatic renal cell carcinoma: report of two cases. Ann Oncol. 2010; 21:1383–1385.
Article30. Sapkota K, Kim S, Park SE, Kim SJ. Detoxified extract of Rhus verniciflua stokes inhibits rotenone-induced apoptosis in human dopaminergic cells, SH-SY5Y. Cell Mol Neurobiol. 2011; 31:213–223.
Article31. Ko JH, Lee SJ, Lim KT. Rhus verniciflua Stokes glycoprotein (36kDa) has protective activity on carbon tetrachloride-induced liver injury in mice. Environ Toxicol Pharmacol. 2006; 22:8–14.
Article32. Stipcevic T, Piljac J, Vanden Berghe D. Effect of different flavonoids on collagen synthesis in human fibroblasts. Plant Foods Hum Nutr. 2006; 61:29–34.
Article33. Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000; 114:480–486.
Article34. Watanabe H, Shimizu T, Nishihira J, Abe R, Nakayama T, Taniguchi M, et al. Ultraviolet A-induced production of matrix metalloproteinase-1 is mediated by macrophage migration inhibitory factor (MIF) in human dermal fibroblasts. J Biol Chem. 2004; 279:1676–1683.
Article35. Kim HH, Lee MJ, Lee SR, Kim KH, Cho KH, Eun HC, et al. Augmentation of UV-induced skin wrinkling by infrared irradiation in hairless mice. Mech Ageing Dev. 2005; 126:1170–1177.
Article36. Krishnamoorthy G, Selvakumar R, Sastry TP, Sadulla S, Mandal AB, Doble M. Experimental and theoretical studies on Gallic acid assisted EDC/NHS initiated crosslinked collagen scaffolds. Mater Sci Eng C Mater Biol Appl. 2014; 43:164–171.
Article37. Takasao N, Tsuji-Naito K, Ishikura S, Tamura A, Akagawa M. Cinnamon extract promotes type I collagen biosynthesis via activation of IGF-I signaling in human dermal fibroblasts. J Agric Food Chem. 2012; 60:1193–1200.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experimental Study on Latent Sensitivity to Rhus Trees
- Protective effect of Rhus verniciflua Stokes extract in an experimental model of post-menopausal osteoporosis
- Urushiol V Suppresses Cell Proliferation and Enhances Antitumor Activity of 5-FU in Human Colon Cancer Cells by Downregulating FoxM1
- Rhus verniciflua Stokes extract suppresses migration and invasion in human gastric adenocarcinoma AGS cells
- Bark Constituents from Mushroom-detoxified Rhus verniciflua Suppress Kainic Acid-induced Neuronal Cell Death in Mouse Hippocampus