Cancer Res Treat.
2017 Apr;49(2):509-517. 10.4143/crt.2016.214.
The Role of Notch1 Signaling in Anaplastic Thyroid Carcinoma
- Affiliations
-
- 1Laboratory of Radiation Pathology, Korea Cancer Center Hospital, Seoul, Korea. tontos016@naver.com
- 2Department of Pathology, Korea Cancer Center Hospital, Seoul, Korea.
- KMID: 2378124
- DOI: http://doi.org/10.4143/crt.2016.214
Abstract
- PURPOSE
The Notch signaling pathway is widely expressed in normal, reactive, and neoplastic tissues; however, its role in thyroid tissues has not been fully elucidated. Therefore, this study was conducted to characterize the expression of the Notch signaling pathway in papillary thyroid cancer (PTC) cells and anaplastic thyroid cancer (ATC) cells.
MATERIALS AND METHODS
Expression of activated Notch1 in ATC and PTC paraffin-embedded tissues was determined by immunohistochemistry. The small interfering RNA techniquewas employed to knock down Notch1 expression in ATC and PTC cell lines.
RESULTS
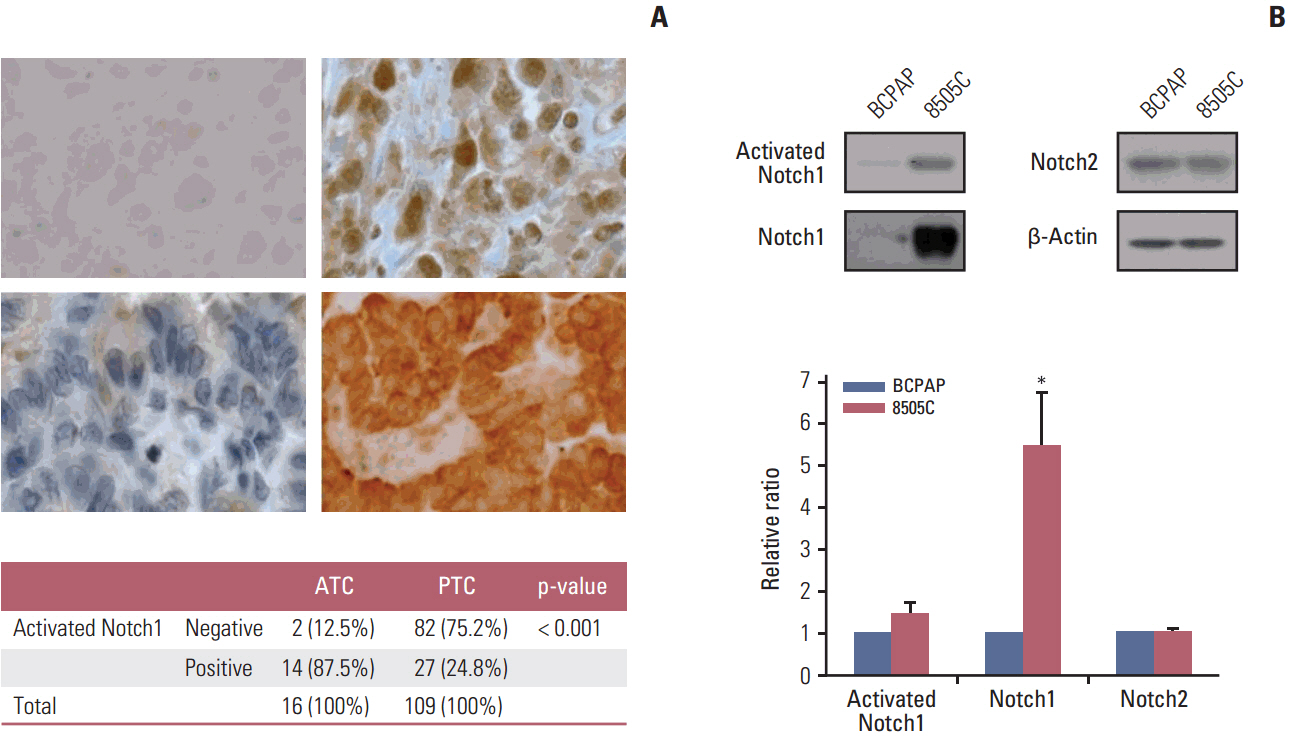
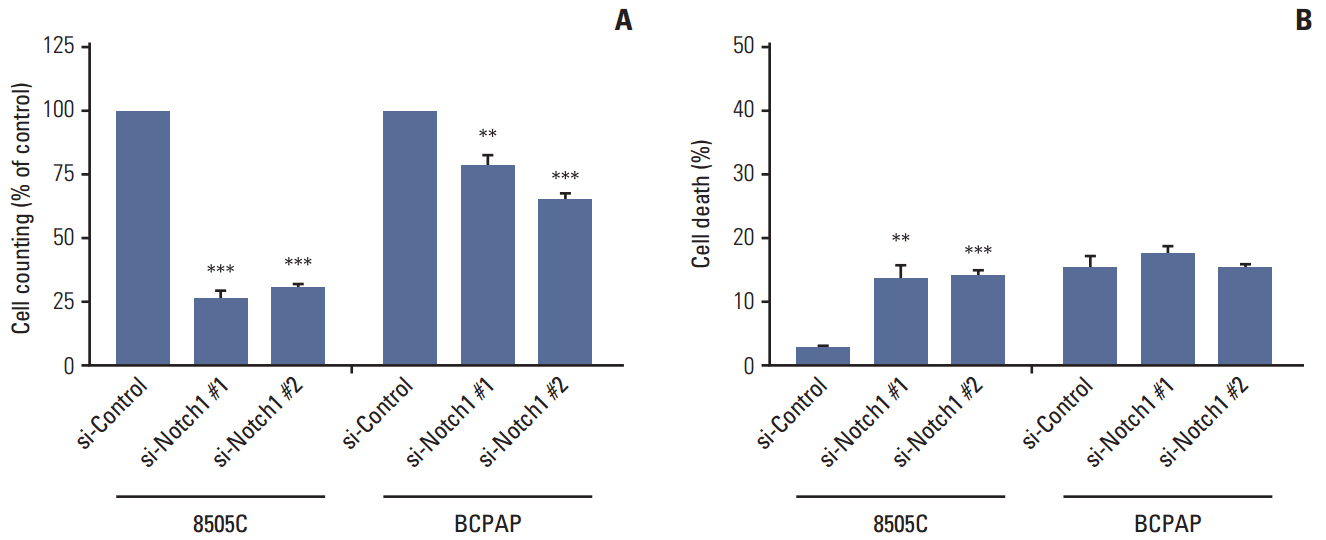
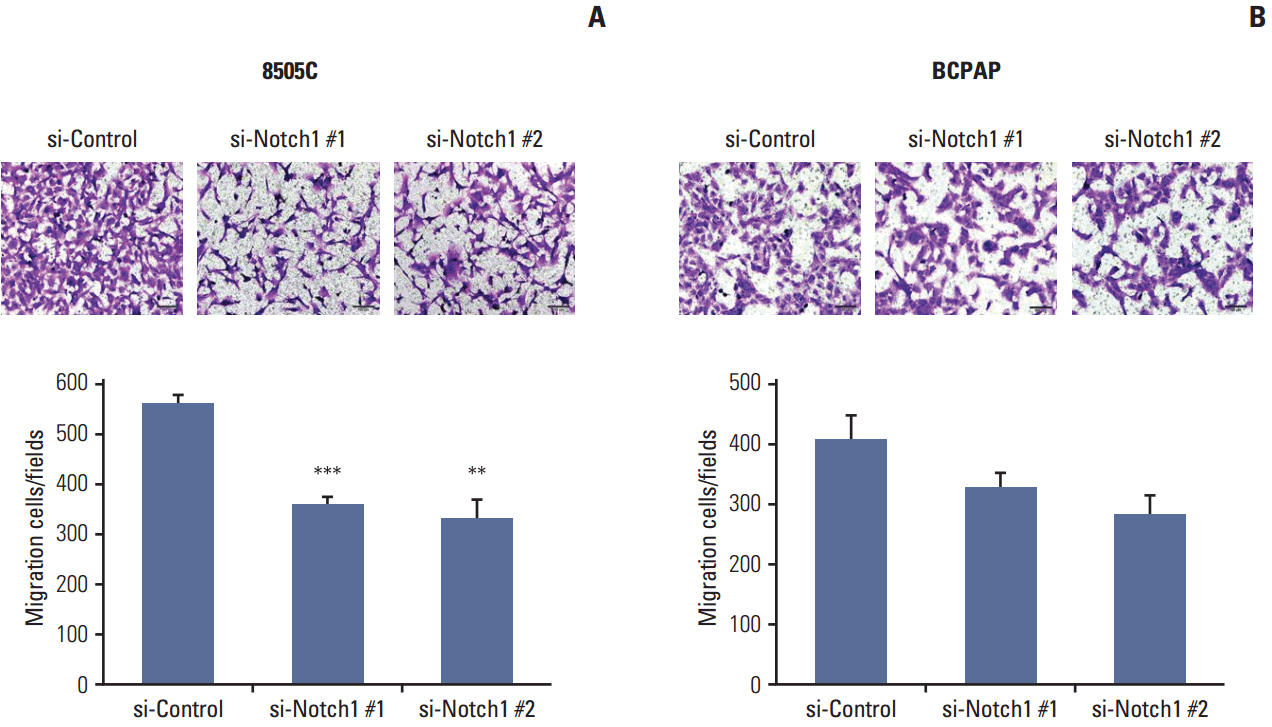
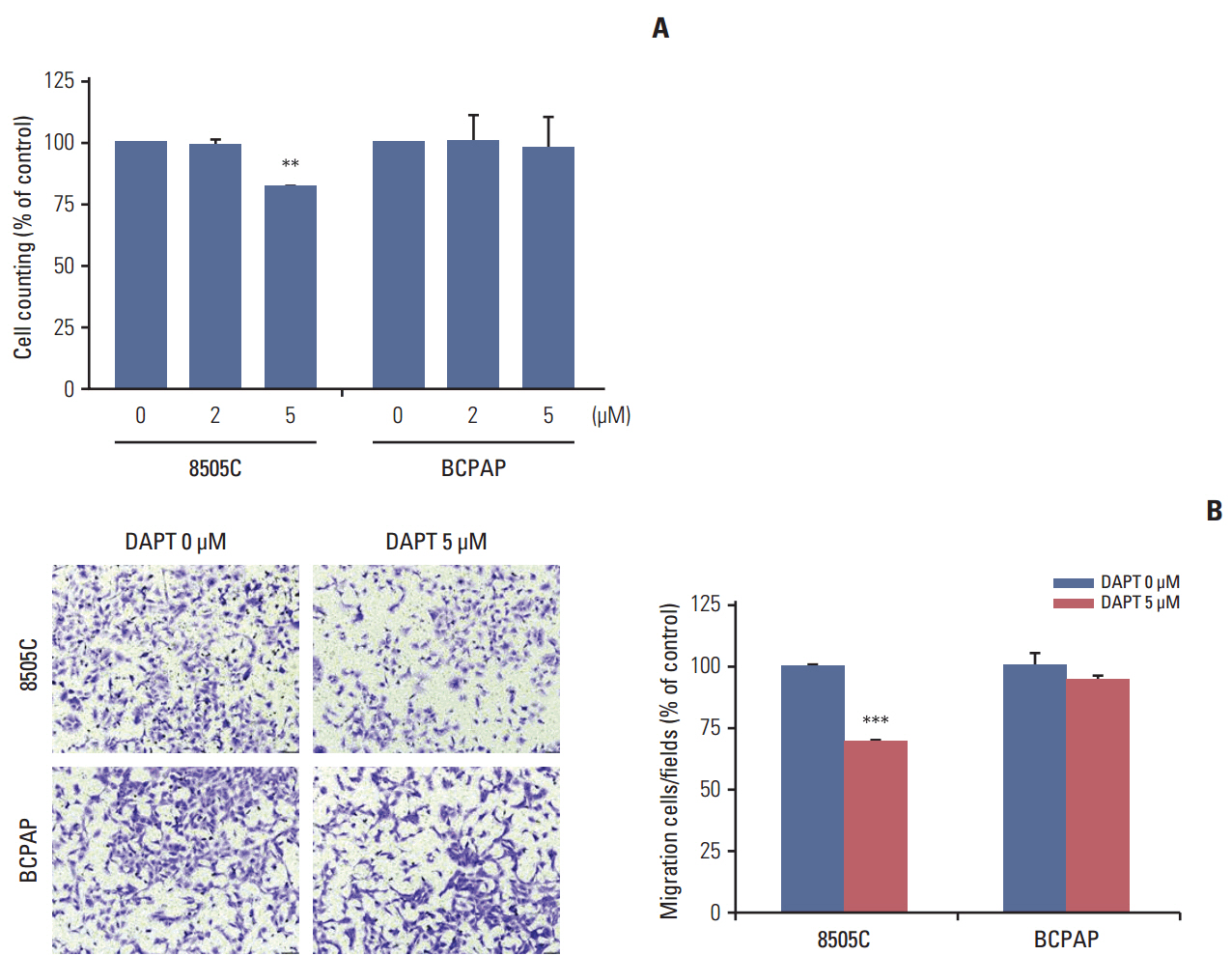
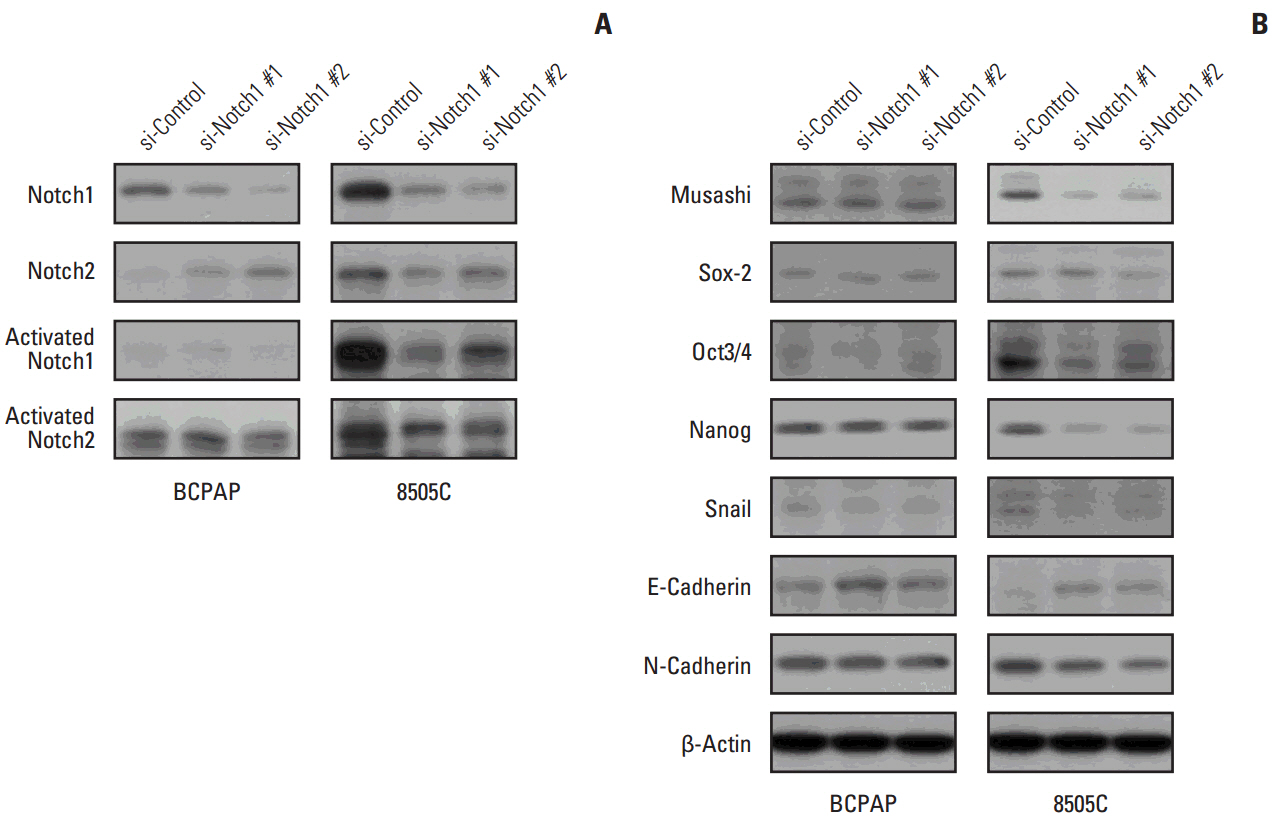
The expression of activated Notch1 was higher in ATC cases than in PTC cases. Inhibition of Notch1 significantly reduced proliferation and migration of ATC cells, but not PTC cells. In addition, inhibition of Notch1 in ATC cells significantly reduced the expression of key markers of epithelial-mesenchymal transition and cancer stem cells. Conversely, changes in the expression of these proteins were not observed in PTC cells.
CONCLUSION
The results of this study suggest that Notch1 expression plays different roles in tumor progression in ATC and PTC cells. We also found that Notch1 expression was significantly related to the highly invasive or proliferative activity of ATC cells.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60:277–300.
Article2. Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008; 21 Suppl 2:S37–43.
Article3. Neff RL, Farrar WB, Kloos RT, Burman KD. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am. 2008; 37:525–38.
Article4. Pasieka JL. Anaplastic thyroid cancer. Curr Opin Oncol. 2003; 15:78–83.
Article5. Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). 2010; 22:486–97.
Article6. Maillard I, Pear WS. Notch and cancer: best to avoid the ups and downs. Cancer Cell. 2003; 3:203–5.
Article7. Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005; 8:709–15.
Article8. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009; 137:216–33.
Article9. Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett. 2006; 580:2860–8.
Article10. Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006; 7:678–89.
Article11. Kanwar R, Fortini ME. Notch signaling: a different sort makes the cut. Curr Biol. 2004; 14:R1043–5.
Article12. Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002; 3:673–84.13. van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005; 435:959–63.14. Cao YW, Wan GX, Sun JP, Cui XB, Hu JM, Liang WH, et al. Implications of the Notch1-Snail/Slug-epithelial to mesenchymal transition axis for lymph node metastasis in infiltrating ductal carcinoma. Kaohsiung J Med Sci. 2015; 31:70–6.
Article15. Gopalakrishnan N, Sivasithamparam ND, Devaraj H. Synergistic association of Notch and NFkappaB signaling and role of Notch signaling in modulating epithelial to mesenchymal transition in colorectal adenocarcinoma. Biochimie. 2014; 107 Pt B:310–8.16. Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006; 66:1517–25.
Article17. Wang Z, Li Y, Kong D, Sarkar FH. The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets. 2010; 11:745–51.
Article18. Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011; 307:26–36.19. Chen W, Cao G, Yuan X, Zhang X, Zhang Q, Zhu Y, et al. Notch-1 knockdown suppresses proliferation, migration and metastasis of salivary adenoid cystic carcinoma cells. J Transl Med. 2015; 13:167.
Article20. Zhang Y, Li B, Ji ZZ, Zheng PS. Notch1 regulates the growth of human colon cancers. Cancer. 2010; 116:5207–18.
Article21. Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006; 66:2778–84.22. Ferretti E, Tosi E, Po A, Scipioni A, Morisi R, Espinola MS, et al. Notch signaling is involved in expression of thyrocyte differentiation markers and is down-regulated in thyroid tumors. J Clin Endocrinol Metab. 2008; 93:4080–7.
Article23. Xiao X, Ning L, Chen H. Notch1 mediates growth suppression of papillary and follicular thyroid cancer cells by histone deacetylase inhibitors. Mol Cancer Ther. 2009; 8:350–6.
Article24. Vasko V, Espinosa AV, Scouten W, He H, Auer H, Liyanarachchi S, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci U S A. 2007; 104:2803–8.
Article25. Su BH, Qu J, Song M, Huang XY, Hu XM, Xie J, et al. NOTCH1 signaling contributes to cell growth, anti-apoptosis and metastasis in salivary adenoid cystic carcinoma. Oncotarget. 2014; 5:6885–95.
Article26. Miele L, Miao H, Nickoloff BJ. NOTCH signaling as a novel cancer therapeutic target. Curr Cancer Drug Targets. 2006; 6:313–23.
Article27. Saad S, Stanners SR, Yong R, Tang O, Pollock CA. Notch mediated epithelial to mesenchymal transformation is associated with increased expression of the Snail transcription factor. Int J Biochem Cell Biol. 2010; 42:1115–22.
Article28. Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, et al. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007; 204:2935–48.
Article29. Buehler D, Hardin H, Shan W, Montemayor-Garcia C, Rush PS, Asioli S, et al. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol. 2013; 26:54–61.
Article30. Jung CW, Han KH, Seol H, Park S, Koh JS, Lee SS, et al. Expression of cancer stem cell markers and epithelial-mesenchymal transition-related factors in anaplastic thyroid carcinoma. Int J Clin Exp Pathol. 2015; 8:560–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DNA Ploidy in Anaplastic Carcinoma of the Thyroid Gland by Image Analysis
- Fine Needle Aspiration Cytology of Anaplastic Carcinoma with Osteoclastlike Giant Cells of the Thyroid
- Effect of growth factors and differentiation inducer DMSO on the anaplastic thyroid carcinoma cell line, SNU-80
- Fine Needle Aspiration Cytology of Anaplastic Carcinoma of the Thyroid with Osteoclast-like Giant Cells : A Case Report
- High Notch1 Expression Correlates with Tumor Stage and Size in Clear Cell Renal Cell Carcinoma