Immune Netw.
2017 Apr;17(2):121-127. 10.4110/in.2017.17.2.121.
Augmented Serum Amyloid A1/2 Mediated by TNF-induced NF-κB in Human Serous Ovarian Epithelial Tumors
- Affiliations
-
- 1Department of Biochemistry and Cancer Biology, Meharry Medical College, Nashville, TN 37208, USA. dson@mmc.edu
- 2Department of Pharmaceutical Sciences, College of Pharmacy, Florida A&M University, Tallahassee, FL 32301, USA.
- 3Department of Obstetrics and Gynecology, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN 37232, USA.
- KMID: 2376870
- DOI: http://doi.org/10.4110/in.2017.17.2.121
Abstract
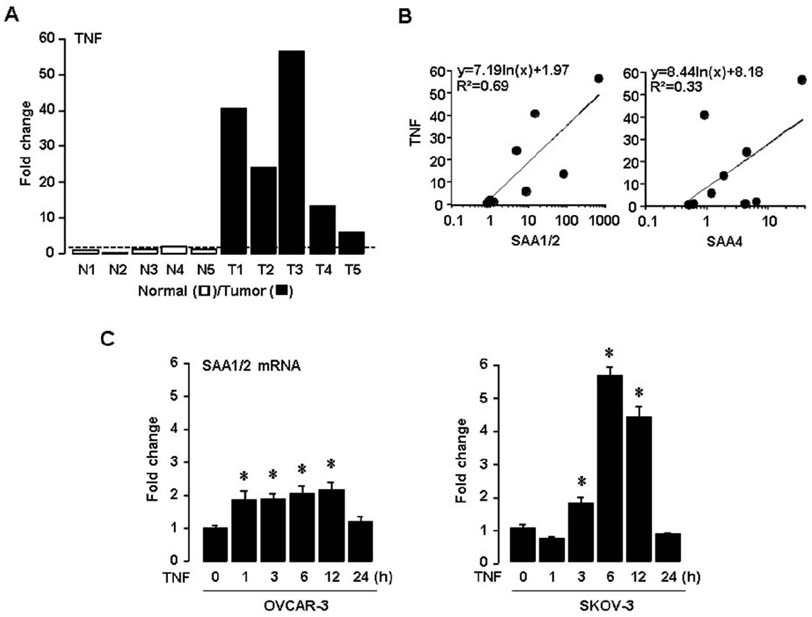
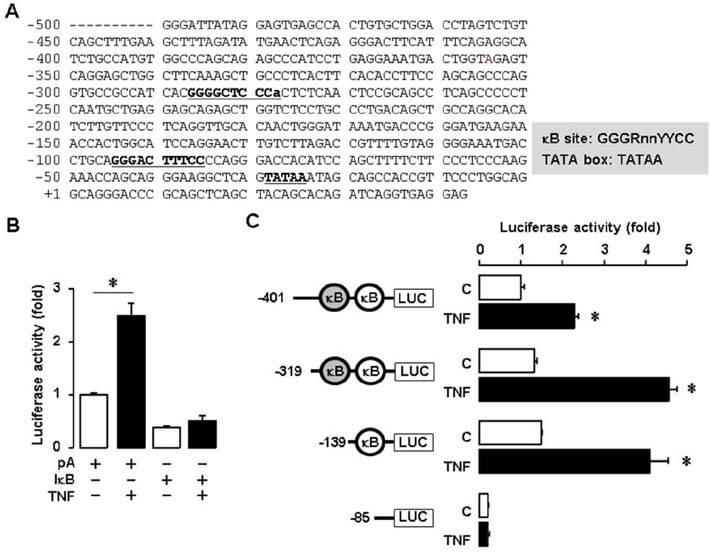
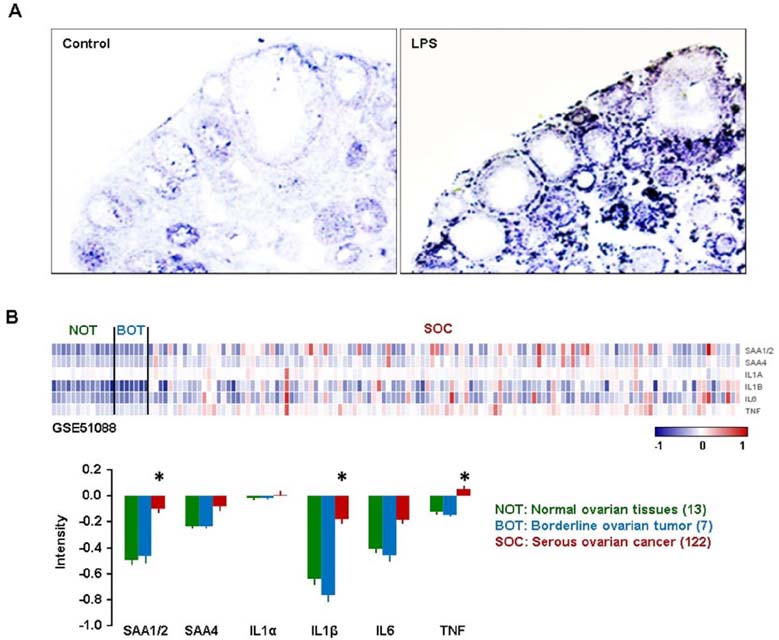
- Tumor necrosis factor-α (TNF) is well known to be involved in the immune system and ovarian inflammation. Ovarian cancer is an inflammation-related malignancy that lacks early screening strategies, resulting in late diagnosis followed by high mortality. Based on our previous data, TNF induced abundant serum amyloid A (SAA), an acute phase protein linked to inflammation, in ovarian granulosal cells. To date, the regulation and expression of SAA in ovarian cancer is not fully elucidated. Here, we investigated the relationship between TNF and SAA by comparing human normal ovarian tissues and serous ovarian tumors. We found that SAA1/2 was significantly expressed in tumor tissues, but no or trace expression levels in normal tissues. TNF was also significantly upregulated in ovarian tumor tissues compared to normal tissues. Moreover, TNF significantly increased SAA1/2 levels in human ovarian cancer cell lines, OVCAR-3 and SKOV-3, in a time-dependent manner. Since the SAA1 promoter contains two nuclear factor (NF)-κB sites, we examined whether TNF regulates SAA1 promoter activity. Deletion analysis revealed that the proximal NF-κB site (−95/−85) played a critical role in regulating TNF-induced SAA1 promoter activity. Within 2 h after intraperitoneal injection of lipopolysaccharide, a product known to stimulate release of TNF, SAA preferably localized to ovarian epithelial cells and the thecal-interstitial layers compared to granulosal cell layers. Based on Gene Expression Omnibus (GEO) database, SAA1/2 and TNF were dominantly expressed in advanced grade ovarian cancer. Taken together, the accumulation of SAA1/2 in ovarian cancer could be mediated by TNF-induced NF-κB activation.
Keyword
MeSH Terms
-
Acute-Phase Proteins
Amyloid*
Cell Line
Delayed Diagnosis
Epithelial Cells
Gene Expression
Humans*
Immune System
Inflammation
Injections, Intraperitoneal
Mass Screening
Mortality
Necrosis
Ovarian Neoplasms
Serum Amyloid A Protein
Tumor Necrosis Factor-alpha
Acute-Phase Proteins
Amyloid
Serum Amyloid A Protein
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article2. Shih IM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004; 164:1511–1518.3. Kobel M, Kalloger SE, Boyd N, McKinney S, Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, Prentice LM, Miller D, Santos J, Swenerton K, Gilks CB, Huntsman D. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008; 5:e232.
Article4. Nowak M, Glowacka E, Szpakowski M, Szyllo K, Malinowski A, Kulig A, Tchorzewski H, Wilczynski J. Proinflammatory and immunosuppressive serum, ascites and cyst fluid cytokines in patients with early and advanced ovarian cancer and benign ovarian tumors. Neuro Endocrinol Lett. 2010; 31:375–383.5. Clendenen TV, Lundin E, Zeleniuch-Jacquotte A, Koenig KL, Berrino F, Lukanova A, Lokshin AE, Idahl A, Ohlson N, Hallmans G, Krogh V, Sieri S, Muti P, Marrangoni A, Nolen BM, Liu M, Shore RE, Arslan AA. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2011; 20:799–810.
Article6. Son DS, Kabir SM, Dong Y, Lee E, Adunyah SE. Characteristics of chemokine signatures elicited by EGF and TNF in ovarian cancer cells. J Inflamm (Lond). 2013; 10:25.
Article7. Ignacio RM, Kabir SM, Lee ES, Adunyah SE, Son DS. NF-kappaB-mediated CCL20 reigns dominantly in CXCR2-driven ovarian cancer progression. PLoS One. 2016; 11:e0164189.8. Dong YL, Kabir SM, Lee ES, Son DS. CXCR2-driven ovarian cancer progression involves upregulation of proinflammatory chemokines by potentiating NF-kappaB activation via EGFR-transactivated Akt signaling. PLoS One. 2013; 8:e83789.9. Dobrzycka B, Terlikowski SJ, Kowalczuk O, Kinalski M. Circulating levels of TNF-alpha and its soluble receptors in the plasma of patients with epithelial ovarian cancer. Eur Cytokine Netw. 2009; 20:131–134.
Article10. Maccio A, Madeddu C. Inflammation and ovarian cancer. Cytokine. 2012; 58:133–147.
Article11. Szlosarek PW, Grimshaw MJ, Kulbe H, Wilson JL, Wilbanks GD, Burke F, Balkwill FR. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol Cancer Ther. 2006; 5:382–390.
Article12. Urieli-Shoval S, Linke RP, Matzner Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol. 2000; 7:64–69.
Article13. Targonska-Stepniak B, Majdan M. Serum amyloid A as a marker of persistent inflammation and an indicator of cardiovascular and renal involvement in patients with rheumatoid arthritis. Mediators Inflamm. 2014; 2014:793628.14. Thorn CF, Lu ZY, Whitehead AS. Regulation of the human acute phase serum amyloid A genes by tumour necrosis factor-alpha, interleukin-6 and glucocorticoids in hepatic and epithelial cell lines. Scand J Immunol. 2004; 59:152–158.
Article15. Urieli-Shoval S, Finci-Yeheskel Z, Dishon S, Galinsky D, Linke RP, Ariel I, Levin M, Ben-Shachar I, Prus D. Expression of serum amyloid a in human ovarian epithelial tumors: implication for a role in ovarian tumorigenesis. J Histochem Cytochem. 2010; 58:1015–1023.
Article16. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 8:e1000412.
Article17. Son DS, Roby KF. Interleukin-1alpha-induced chemokines in mouse granulosa cells: impact on keratinocyte chemoattractant chemokine, a CXC subfamily. Mol Endocrinol. 2006; 20:2999–3013.
Article18. Son DS, Terranova PF, Roby KF. Interaction of adenosine 3',5'-cyclic monophosphate and tumor necrosis factor-alpha on serum amyloid A3 expression in mouse granulosa cells: dependence on CCAAT-enhancing binding protein-beta isoform. Endocrinology. 2010; 151:3407–3419.
Article19. Perez-Llamas C, Lopez-Bigas N. Gitools: analysis and visualisation of genomic data using interactive heat-maps. PLoS One. 2011; 6:e19541.
Article20. Son DS, Roby KF, Terranova PF. Tumor necrosis factor-alpha induces serum amyloid A3 in mouse granulosa cells. Endocrinology. 2004; 145:2245–2252.
Article21. Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013; 12:86.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory mechanism on NF-kB transactivation by dexamethasone in pulmonary epithelial cells
- Effect of Transcription Factor Decoy for NF-κB on the TNF-α Induced Cytokine and ICAM-1 Expression in Cultured HaCaT cells
- Pro-inflammatory cytokine expression through NF-kappaB/IkappaB pathway in lung epithelial cells
- Expression of p53, c-myc, Transforming Growth Factor-alpha and -beta in Human Epithelial Ovarian Tumors
- Triptolide-induced Transrepression of IL-8 NF-kappaB in Lung Epithelial Cells