Immune Netw.
2017 Apr;17(2):103-109. 10.4110/in.2017.17.2.103.
Detection of Autoantibodies against Aquaporin-1 in the Sera of Patients with Primary Sjögren's Syndrome
- Affiliations
-
- 1School of Dentistry and Dental Research Institute, Seoul National University, Seoul 03080, Korea. youngnim@snu.ac.kr
- 2Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul 06591, Korea.
- 3College of Medicine, Seoul National University, Seoul 03080, Korea.
- KMID: 2376867
- DOI: http://doi.org/10.4110/in.2017.17.2.103
Abstract
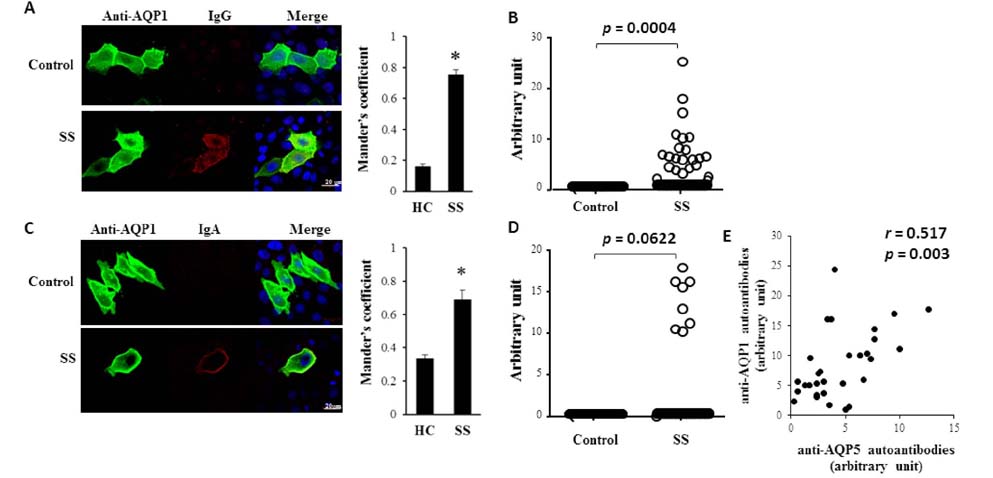
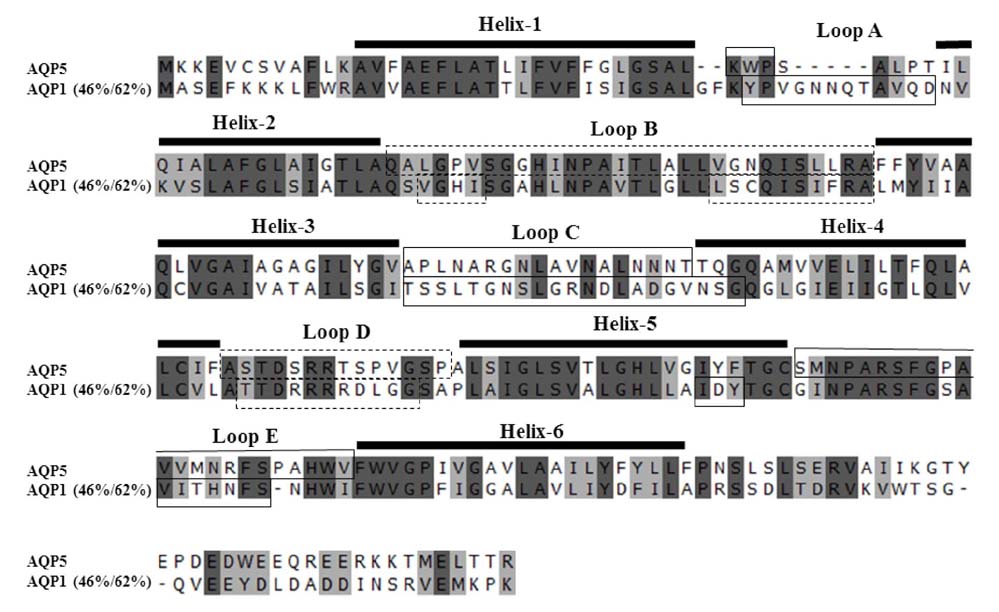
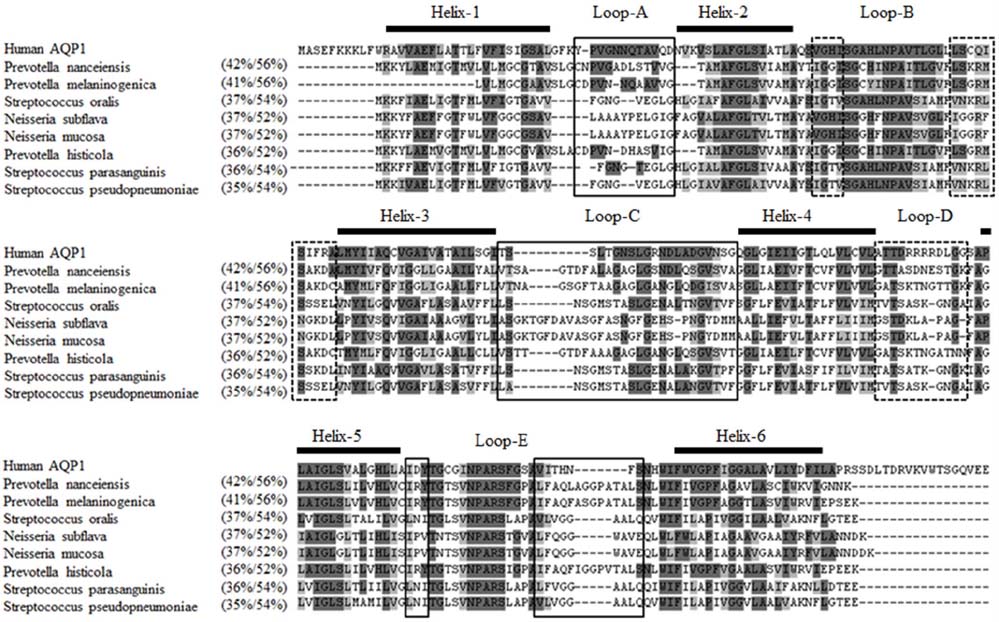
- The pathophysiology of glandular dysfunction in Sjögren's syndrome (SS) has not been fully elucidated. Previously, we reported the presence of autoantibodies to AQP-5 in patients with SS, which was associated with a low resting salivary flow. The purpose of this study was to investigate the presence of anti-AQP1 autoantibodies. To detect anti-AQP1 autoantibodies, cell-based indirect immunofluorescence assay was developed using MDCK cells that overexpressed human AQP1. By screening 112 SS and 52 control sera, anti-AQP1 autoantibodies were detected in 27.7% of the SS but in none of the control sera. Interestingly, the sera that were positive for anti-AQP1 autoantibodies also contained anti-AQP5 autoantibodies in the previous study. Different from anti-AQP5 autoantibodies, the presence of anti-AQP1 autoantibodies was not associated with the salivary flow rate. Although anti-AQP1 autoantibodies are not useful as a diagnostic marker, the presence of autoantibodies to AQP1 may be an obstacle to AQP1 gene therapy for SS.
MeSH Terms
Figure
Reference
-
1. Luciano N, Valentini V, Calabro A, Elefante E, Vitale A, Baldini C, Bartoloni E. One year in review 2015: Sjogren's syndrome. Clin Exp Rheumatol. 2015; 33:259–271.2. Fox RI. Sjogren's syndrome. Lancet. 2005; 366:321–331.3. Robinson CP, Brayer J, Yamachika S, Esch TR, Peck AB, Stewart CA, Peen E, Jonsson R, Humphreys-Beher MG. Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjogren's syndrome. Proc Natl Acad Sci U S A. 1998; 95:7538–7543.
Article4. Smith PM, Dawson LJ. Autoantibodies and Sjogren's syndrome: a physiologist's perspective. Curr Pharm Biotechnol. 2012; 13:2063–2070.
Article5. Lee BH, Gauna AE, Perez G, Park YJ, Pauley KM, Kawai T, Cha S. Autoantibodies against muscarinic type 3 receptor in Sjogren's syndrome inhibit aquaporin 5 trafficking. PLoS One. 2013; 8:e53113.6. Proctor GB, Carpenter GH. Salivary secretion: mechanism and neural regulation. Monogr Oral Sci. 2014; 24:14–29.
Article7. Catalan MA, Nakamoto T, Melvin JE. The salivary gland fluid secretion mechanism. J Med Invest. 2009; 56:Suppl. 192–196.
Article8. Delporte C, Bryla A, Perret J. Aquaporins in Salivary Glands: From Basic Research to Clinical Applications. Int J Mol Sci. 2016; 17:E166.
Article9. Beroukas D, Hiscock J, Gannon BJ, Jonsson R, Gordon TP, Waterman SA. Selective down-regulation of aquaporin-1 in salivary glands in primary Sjogren's syndrome. Lab Invest. 2002; 82:1547–1552.
Article10. Proctor GB. The physiology of salivary secretion. Periodontol 2000. 2016; 70:11–25.
Article11. Mobasheri A, Marples D. Expression of the AQP-1 water channel in normal human tissues: a semiquantitative study using tissue microarray technology. Am J Physiol Cell Physiol. 2004; 286:C529–C537.
Article12. Gresz V, Horvath A, Gera I, Nielsen S, Zelles T. Immunolocalization of AQP5 in resting and stimulated normal labial glands and in Sjogren's syndrome. Oral Dis. 2015; 21:e114–e120.13. Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999; 274:20071–20074.
Article14. Ring T, Kallenbach M, Praetorius J, Nielsen S, Melgaard B. Successful treatment of a patient with primary Sjogren's syndrome with Rituximab. Clin Rheumatol. 2006; 25:891–894.
Article15. Lai Z, Yin H, Cabrera-Perez J, Guimaro MC, Afione S, Michael DG, Glenton P, Patel A, Swaim WD, Zheng C, Nguyen CQ, Nyberg F, Chiorini JA. Aquaporin gene therapy corrects Sjogren's syndrome phenotype in mice. Proc Natl Acad Sci U S A. 2016; 113:5694–5699.16. Delporte C, O'Connell BC, He X, Lancaster HE, O'Connell AC, Agre P, Baum BJ. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci U S A. 1997; 94:3268–3273.
Article17. Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Goldsmith CM, Burbelo PD, Citrin DE, Mitchell JB, Nottingham LK, Rudy SF, Van WC, Whatley MA, Brahim JS, Chiorini JA, Danielides S, Turner RJ, Patronas NJ, Chen CC, Nikolov NP, Illei GG. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci U S A. 2012; 109:19403–19407.
Article18. Alam J, Koh JH, Kim N, Kwok SK, Park SH, Song YW, Park K, Choi Y. Detection of autoantibodies against aquaporin-5 in the sera of patients with primary Sjogren's syndrome. Immunol Res. 2016; 64:848–856.
Article19. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61:554–558.
Article20. Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, Schiodt M, Umehara H, Vivino F, Zhao Y, Dong Y, Greenspan D, Heidenreich AM, Helin P, Kirkham B, Kitagawa K, Larkin G, Li M, Lietman T, Lindegaard J, McNamara N, Sack K, Shirlaw P, Sugai S, Vollenweider C, Whitcher J, Wu A, Zhang S, Zhang W, Greenspan J, Daniels T. American college of rheumatology classification criteria for Sjogren's syndrome: a data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res(Hoboken). 2012; 64:475–487.
Article21. Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res. 1982; 61:1158–1162.
Article22. Kingston RE, Chen CA, Okayama H. Calcium phosphate transfection. In : Bonifacino JS, Harford JB, Lippincott-Schwartz J, Yamada KM, Dasso M, Nadkarni A, editors. Current protocols in cell biology. John Wiley and sons, Inc.;2003. p. 19p. 20.3p. 20.3.1–20.3.8.
Article23. Jarius S, Probst C, Borowski K, Franciotta D, Wildemann B, Stoecker W, Wandinger KP. Standardized method for the detection of antibodies to aquaporin-4 based on a highly sensitive immunofluorescence assay employing recombinant target antigen. J Neurol Sci. 2010; 291:52–56.
Article24. Long Y, Zheng Y, Shan F, Chen M, Fan Y, Zhang B, Gao C, Gao Q, Yang N. Development of a cell-based assay for the detection of anti-aquaporin 1 antibodies in neuromyelitis optica spectrum disorders. J Neuroimmunol. 2014; 273:103–110.
Article25. Tzartos JS, Stergiou C, Kilidireas K, Zisimopoulou P, Thomaidis T, Tzartos SJ. Anti-aquaporin-1 autoantibodies in patients with neuromyelitis optica spectrum disorders. PLoS One. 2013; 8:e74773.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Treatment with Steroid and Hydrochloroquine of Thrombocytopenia in Primary Sjögren's Syndrome
- Longitudinal Changes of the European League Against Rheumatism Sjögren's Syndrome Patient Reported Index in Korean Patients with Primary Sjögren's Syndrome
- A Case of Sjögren-Larsson Syndrome
- Rehabilitation using twin-stage method for a Sjögren's syndrome patient with severe discoloration and attrition on upper and lower anterior teeth
- Renal Tubular Acidosis in Patients with Primary Sjögren's Syndrome