Yonsei Med J.
2016 Jul;57(4):1006-1015. 10.3349/ymj.2016.57.4.1006.
Preclinical Study of Cell Therapy for Osteonecrosis of the Femoral Head with Allogenic Peripheral Blood-Derived Mesenchymal Stem Cells
- Affiliations
-
- 1Key Laboratory of Cell Engineering of Guizhou Province, The Affiliated Hospital of Zunyi Medical College, Zunyi, Guizhou, China. oceanzt@163.com
- 2Department of Human Anatomy, Zunyi Medical College, Zunyi, Guizhou, China.
- 3Department of Bone and Joint Surgery, The Affiliated Hospital of Zunyi Medical College, Zunyi, Guizhou, China.
- KMID: 2374136
- DOI: http://doi.org/10.3349/ymj.2016.57.4.1006
Abstract
- PURPOSE
To explore the value of transplanting peripheral blood-derived mesenchymal stem cells from allogenic rabbits (rPBMSCs) to treat osteonecrosis of the femoral head (ONFH).
MATERIALS AND METHODS
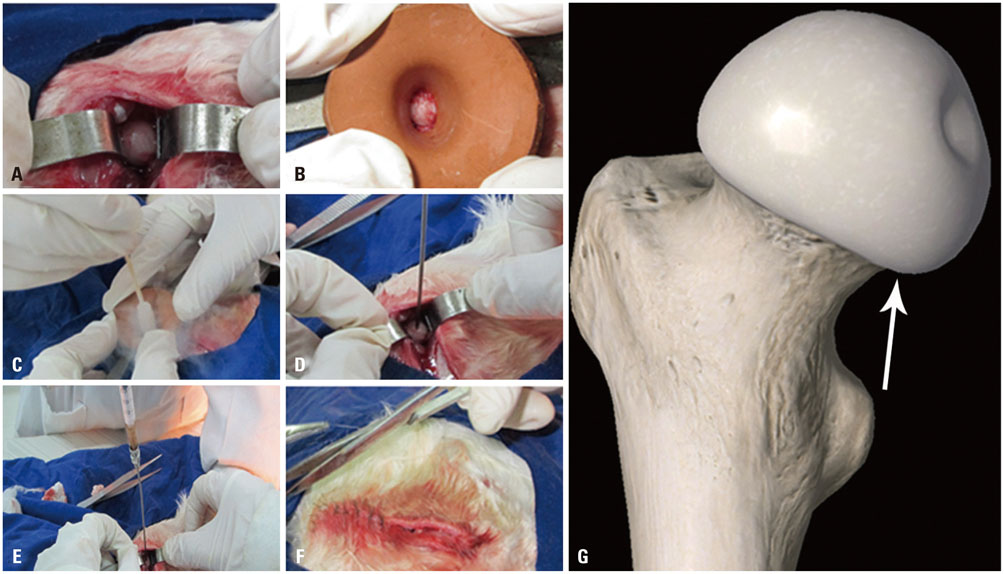
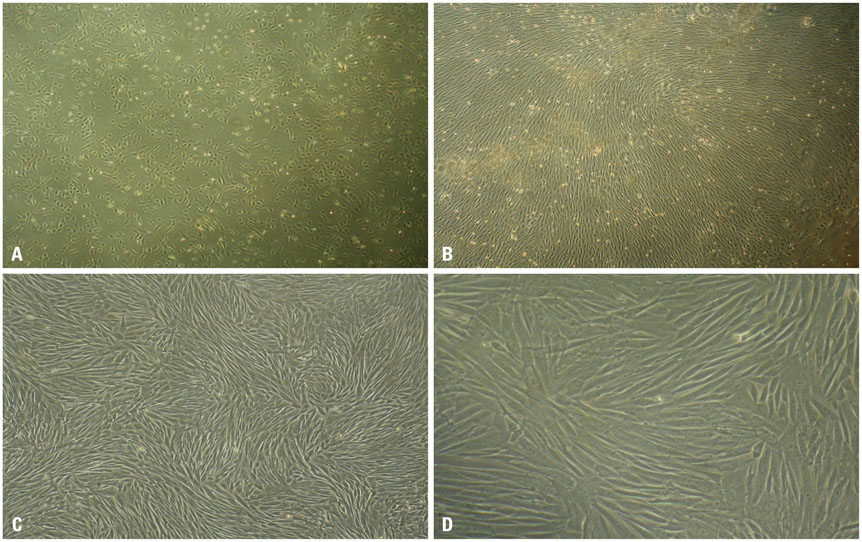
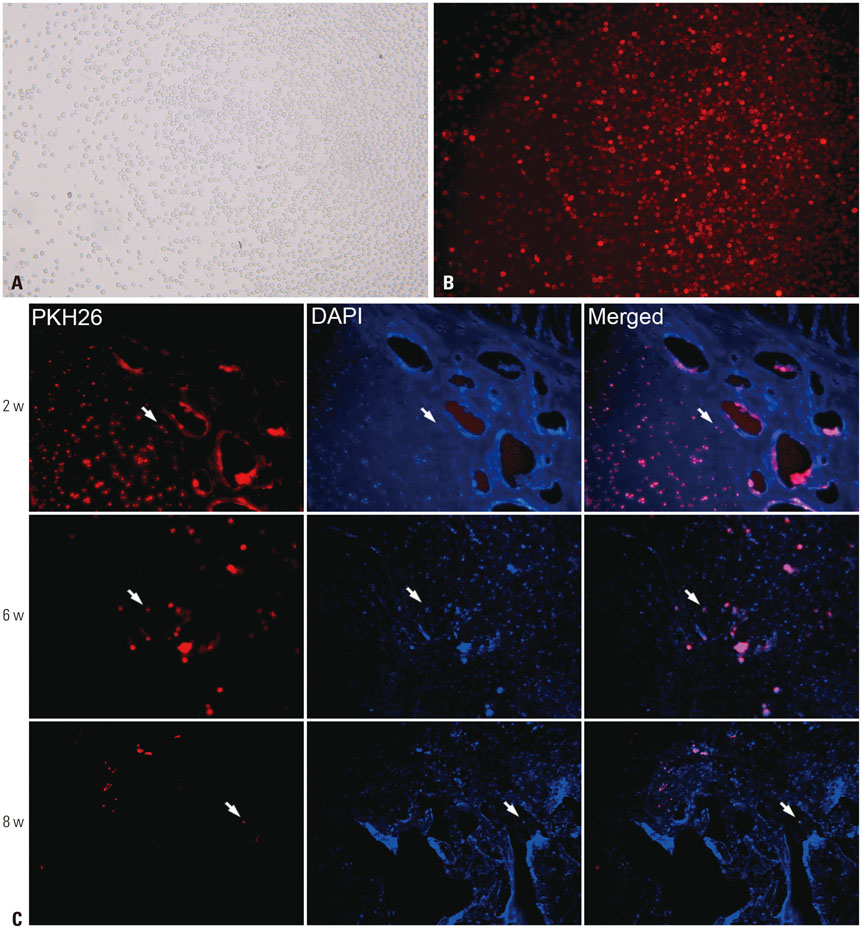
rPBMSCs were separated/cultured from peripheral blood after granulocyte colony-stimulating factor mobilization. Afterwards, mobilized rPBMSCs from a second passage labeled with PKH26 were transplanted into rabbit ONFH models, which were established by liquid nitrogen freezing, to observe the effect of rPBMSCs on ONFH repair. Then, the mRNA expressions of BMP-2 and PPAR-γ in the femoral head were assessed by RT-PCR.
RESULTS
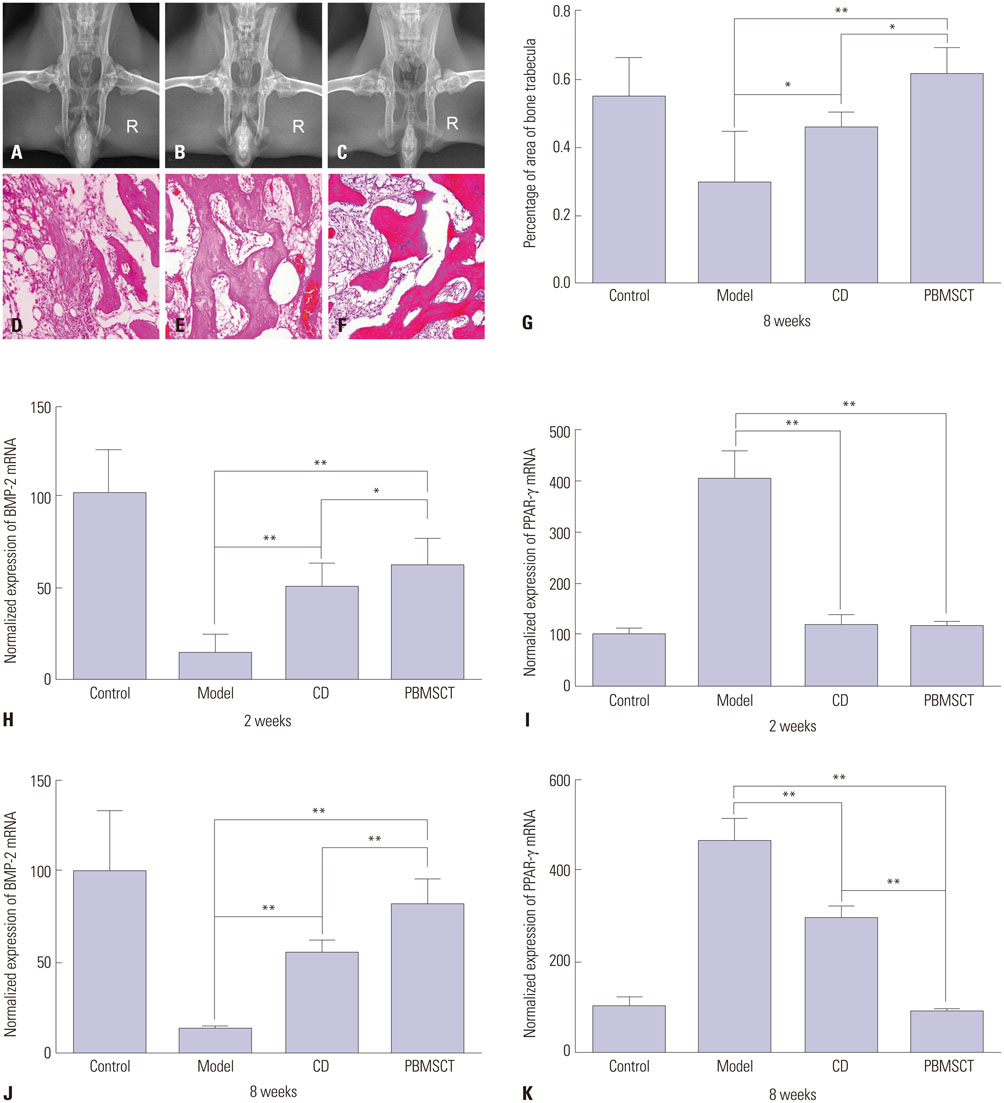
After mobilization, the cultured rPBMSCs expressed mesenchymal markers of CD90, CD44, CD29, and CD105, but failed to express CD45, CD14, and CD34. The colony forming efficiency of mobilized rPBMSCs ranged from 2.8 to 10.8 per million peripheral mononuclear cells. After local transplantation, survival of the engrafted cells reached at least 8 weeks. Therein, BMP-2 was up-regulated, while PPAR-γ mRNA was down-regulated. Additionally, bone density and bone trabeculae tended to increase gradually.
CONCLUSION
We confirmed that local transplantation of rPBMSCs benefits ONFH treatment and that the beneficial effects are related to the up-regulation of BMP-2 expression and the down-regulation of PPAR-γ expression.
Keyword
MeSH Terms
-
Animals
Blood Cells/*cytology
Bone Morphogenetic Protein 2/genetics
*Cell- and Tissue-Based Therapy
Femur Head Necrosis/metabolism/*pathology/*therapy
Gene Expression Regulation
*Mesenchymal Stem Cell Transplantation
Mesenchymal Stromal Cells/*cytology
Osteonecrosis/*pathology/*therapy
PPAR gamma/genetics
Rabbits
Transplantation, Homologous
Bone Morphogenetic Protein 2
PPAR gamma
Figure
Reference
-
1. Smith DW. Is avascular necrosis of the femoral head the result of inhibition of angiogenesis? Med Hypotheses. 1997; 49:497–500.
Article2. Kang JS, Moon KH, Kim BS, Kwon DG, Shin SH, Shin BK, et al. Clinical results of auto-iliac cancellous bone grafts combined with implantation of autologous bone marrow cells for osteonecrosis of the femoral head: a minimum 5-year follow-up. Yonsei Med J. 2013; 54:510–515.
Article3. Bakhshi H, Rasouli MR, Parvizi J. Can local Erythropoietin administration enhance bone regeneration in osteonecrosis of femoral head? Med Hypotheses. 2012; 79:154–156.
Article4. Sun W, Li Z, Gao F, Shi Z, Zhang Q, Guo W. Recombinant human bone morphogenetic protein-2 in debridement and impacted bone graft for the treatment of femoral head osteonecrosis. PLoS One. 2014; 9:e100424.
Article5. Gemma G, Khan M, Haddad FS. Why do total hip replacements fail? Orthop Trauma. 2015; 29:79–85.6. Avisar E, Elvey MH, Bar-Ziv Y, Tamir E, Agar G. Severe vascular complications and intervention following elective total hip and knee replacement: a 16-year retrospective analysis. J Orthop. 2015; 12:151–155.
Article7. Miric A, Inacio MC, Kelly MP, Namba RS. Are nonagenarians too old for total hip arthroplasty? An evaluation of morbidity and mortality within a total joint replacement registry. J Arthroplasty. 2015; 30:1324–1327.
Article8. Ekmekci Y, Keven K, Akar N, Egin Y, Sengul S, Kutlay S, et al. Thrombophilia and avascular necrosis of femoral head in kidney allograft recipients. Nephrol Dial Transplant. 2006; 21:3555–3558.
Article9. Bouamar R, Koper JW, van Rossum EF, Weimar W, van Gelder T. Polymorphisms of the glucocorticoid receptor and avascular necrosis of the femoral heads after treatment with corticosteroids. NDT Plus. 2009; 2:384–386.
Article10. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006; 24:150–154.
Article11. Fukuda S, Hagiwara S, Fukuda S, Yakabe R, Suzuki H, Yabe SG, et al. Safety assessment of bone marrow derived MSC grown in platelet-rich plasma. Regen Ther. 2015; 1:72–79.
Article12. Jones KB, Seshadri T, Krantz R, Keating A, Ferguson PC. Cell-based therapies for osteonecrosis of the femoral head. Biol Blood Marrow Transplant. 2008; 14:1081–1087.
Article13. Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One. 2014; 9:e115963.
Article14. Pak J, Lee JH, Jeon JH, Lee SH. Complete resolution of avascular necrosis of the human femoral head treated with adipose tissuederived stem cells and platelet-rich plasma. J Int Med Res. 2014; 42:1353–1362.
Article15. Pak J. Autologous adipose tissue-derived stem cells induce persistent bone-like tissue in osteonecrotic femoral heads. Pain Physician. 2012; 15:75–85.16. Liu XH, Zhang T, Fang N, Zhang Q, Huang Y, Liu JW, et al. [Verification of multipotential mesenchymal stem cell presence in peripheral blood of rabbits]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013; 21:193–197.17. Krstić J, Obradović H, Jauković A, Okić-Ðorđević I, Trivanović D, Kukolj T, et al. Urokinase type plasminogen activator mediates Interleukin-17-induced peripheral blood mesenchymal stem cell motility and transendothelial migration. Biochim Biophys Acta. 2015; 1853:431–444.
Article18. Zhou Y, Singh AK, Hoyt RF Jr, Wang S, Yu Z, Hunt T, et al. Regulatory T cells enhance mesenchymal stem cell survival and proliferation following autologous cotransplantation in ischemic myocardium. J Thorac Cardiovasc Surg. 2014; 148:1131–1137.
Article19. Liu XH, Zhang T, Zhang Q, Liu JW, Liu ZL, Tang NN. Colony forming capacity and neuron differentiation of rabbit peripheral bloodderived mesenchymal stem cells after G-CSF mobilization. J Xi'an Jiaotong Univ (Med Sci). 2014; 35:26–29.20. Zhou ZL, Zhang Q, Peng JC, Huang Y, Tang NN, Zhang T. Novel liquid nitrogen freezing method for establishing a rabbit model of femoral head osteonecrosis and its reliability evaluation. Acta Anatomica Sinica. 2012; 43:284–288.21. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008; 3:1101–1108.
Article22. Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000; 2:477–488.
Article23. Wan C, He Q, Li G. Allogenic peripheral blood derived mesenchymal stem cells (MSCs) enhance bone regeneration in rabbit ulna critical-sized bone defect model. J Orthop Res. 2006; 24:610–618.
Article24. Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009; 206:2483–2496.
Article25. Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford). 2008; 47:126–131.
Article26. Wen Q, Ma L, Chen YP, Yang L, Luo W, Wang XN. A rabbit model of hormone-induced early avascular necrosis of the femoral head. Biomed Environ Sci. 2008; 21:398–403.
Article27. Yang C, Yang SH, Du JY, Li J, Xu WH, Xiong YF. Basic fibroblast growth factor gene transfection to enhance the repair of avascular necrosis of the femoral head. Chin Med Sci J. 2004; 19:111–115.28. Wen Q, Ma L, Chen YP, Yang L, Luo W, Wang XN. A rabbit model of hormone-induced early avascular necrosis of the femoral head. Biomed Environ Sci. 2008; 21:398–403.
Article29. Hernigou P, Flouzat-Lachaniette CH, Delambre J, Poignard A, Allain J, Chevallier N, et al. Osteonecrosis repair with bone marrow cell therapies: state of the clinical art. Bone. 2015; 70:102–109.
Article30. Aoyama T, Fujita Y, Madoba K, Nankaku M, Yamada M, Tomita M, et al. Rehabilitation program after mesenchymal stromal cell transplantation augmented by vascularized bone grafts for idiopathic osteonecrosis of the femoral head: a preliminary study. Arch Phys Med Rehabil. 2015; 96:532–539.
Article31. Calori GM, Mazza E, Colombo M, Mazzola S, Mineo GV, Giannoudis PV. Treatment of AVN using the induction chamber technique and a biological-based approach: indications and clinical results. Injury. 2014; 45:369–373.
Article32. Peric M, Dumic-Cule I, Grcevic D, Matijasic M, Verbanac D, Paul R, et al. The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone. 2015; 70:73–86.
Article33. Rosset P, Deschaseaux F, Layrolle P. Cell therapy for bone repair. Orthop Traumatol Surg Res. 2014; 100:1 Suppl. S107–S112.
Article34. Shi XM, Blair HC, Yang X, McDonald JM, Cao X. Tandem repeat of C/EBP binding sites mediates PPARgamma2 gene transcription in glucocorticoid-induced adipocyte differentiation. J Cell Biochem. 2000; 76:518–527.
Article35. Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, et al. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996; 50:1087–1094.36. Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res. 1998; (346):26–37.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Mesenchymal Stem Cells in Patients with Nontraumatic Osteonecrosis of the Femoral Head
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Stem Cell Therapy in Osteonecrosis of the Femoral Head
- Stem Cell Therapy for the Treatment of Hip Osteonecrosis: A 30-Year Review of Progress
- The Case Report of a Child with High-Risk Acute Lymphoblastic Leukemia, Treated with Allogenic Peripheral Blood Stem Cell Transplantation