Ann Lab Med.
2016 Jul;36(4):342-352. 10.3343/alm.2016.36.4.342.
Liquid Chromatography-Mass Spectrometry-Based In Vitro Metabolic Profiling Reveals Altered Enzyme Expressions in Eicosanoid Metabolism
- Affiliations
-
- 1Materials and Life Science Research Division, Korea Institute of Science and Technology, Seoul, Korea. mh_choi@kist.re.kr mdhsseo@korea.ac.kr
- 2Department of Chemistry, Yonsei University, Seoul, Korea.
- 3Cardiovascular Center, Korea University Guro Hospital, Seoul, Korea. mh_choi@kist.re.kr mdhsseo@korea.ac.kr
- KMID: 2373554
- DOI: http://doi.org/10.3343/alm.2016.36.4.342
Abstract
- BACKGROUND
Eicosanoids are metabolites of arachidonic acid that are rapidly biosynthesized and degraded during inflammation, and their metabolic changes reveal altered enzyme expression following drug treatment. We developed an eicosanoid profiling method and evaluated their changes on drug treatment.
METHODS
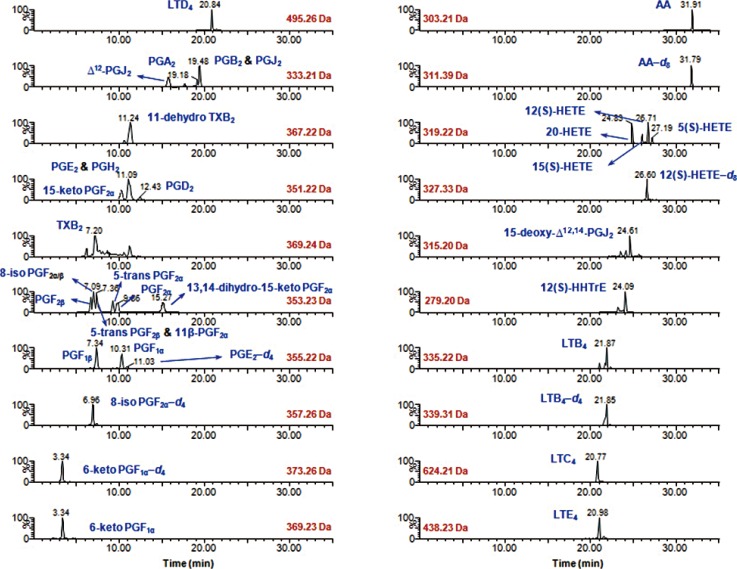
Simultaneous quantitative profiling of 32 eicosanoids in liver S9 fractions obtained from rabbits with carrageenan-induced inflammation was performed and validated by liquid chromatography-mass spectrometry coupled to anion-exchange solid-phase purification.
RESULTS
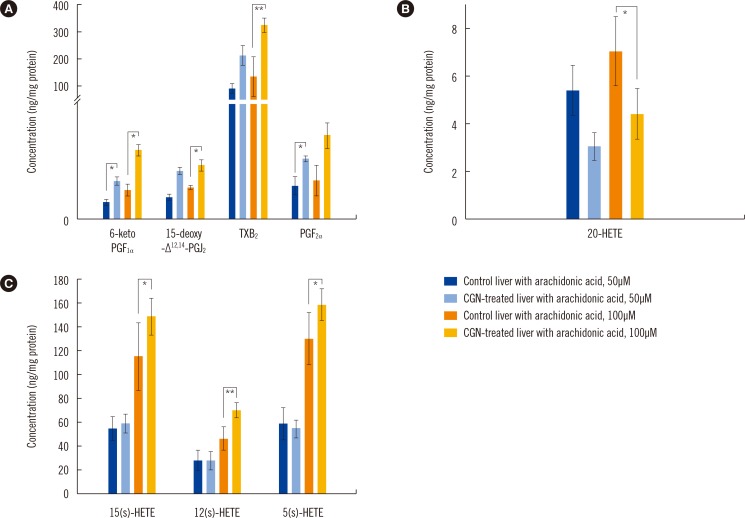
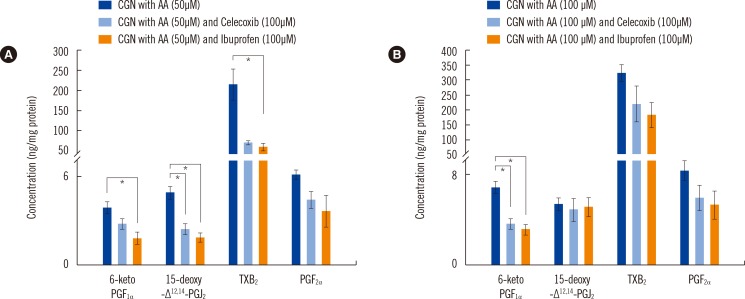
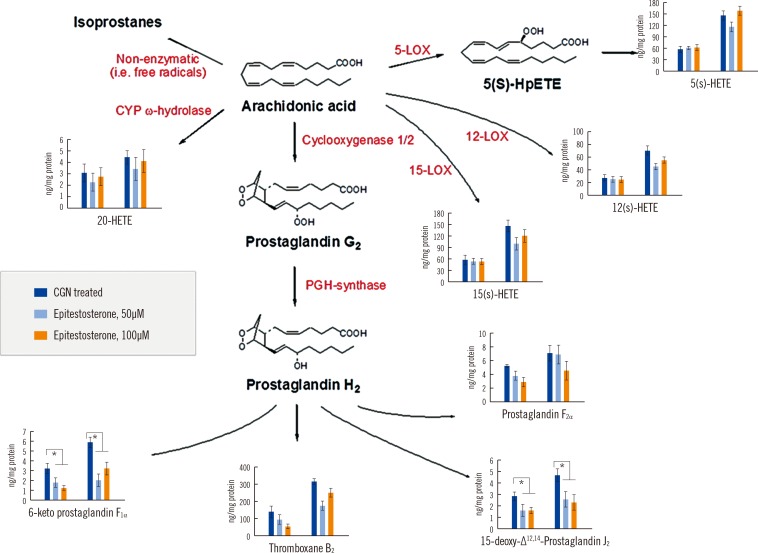
The limit of quantification for the devised method ranged from 0.5 to 20.0 ng/mg protein, and calibration linearity was achieved (R 2>0.99). The precision (% CV) and accuracy (% bias) ranged from 4.7 to 10.3% and 88.4 to 110.9%, respectively, and overall recoveries ranged from 58.0 to 105.3%. Our method was then applied and showed that epitestosterone treatment reduced the levels of all eicosanoids that were generated by cyclooxygenases and lipoxygenases.
CONCLUSIONS
Quantitative eicosanoid profiling combined with in vitro metabolic assays may be useful for evaluating metabolic changes affected by drugs during eicosanoid metabolism.
Keyword
MeSH Terms
-
Animals
Carrageenan/toxicity
*Chromatography, High Pressure Liquid/standards
Cytokines/blood
Disease Models, Animal
Eicosanoids/*analysis/metabolism/standards
Inflammation/etiology/metabolism
Male
Rabbits
Reference Standards
Solid Phase Extraction
*Tandem Mass Spectrometry/standards
Carrageenan
Cytokines
Eicosanoids
Figure
Reference
-
1. Musiek ES, Milne GL, McLaughlin B, Morrow JD. Cyclopentenone eicosanoids as mediators of neurodegeneration: a pathogenic mechanism of oxidative stress-mediated and cyclooxygenase-mediated neurotoxicity. Brain Pathol. 2005; 15:149–158. PMID: 15912888.
Article2. Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008; 9:162–176. PMID: 18216772.
Article3. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008; 8:349–361. PMID: 18437155.
Article4. Balazy M. Eicosanomics: targeted lipidomics of eicosanoids in biological systems. Prostaglandins Other Lipid Mediat. 2004; 73:173–180. PMID: 15287150.
Article5. Theken KN, Deng Y, Kannon MA, Miller TM, Poloyac SM, Lee CR. Activation of the acute inflammatory response alters cytochrome P450 expression and eicosanoid metabolism. Drug Metab Dispos. 2011; 39:22–29. PMID: 20947618.
Article6. Han X, Gross RW. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J Lipid Res. 2003; 44:1071–1079. PMID: 12671038.
Article7. Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997; 36:1–21. PMID: 9373618.
Article8. Masoodi M, Volmer DA. Comprehensive quantitative determination of PUFA-related bioactive lipids for functional lipidomics using high-resolution mass spectrometry. Methods Mol Biol. 2014; 1198:221–232. PMID: 25270932.
Article9. Buczynski MW, Dumlao DS, Dennis EA. Thematic review series: proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009; 50:1015–1038. PMID: 19244215.
Article10. Haeggström JZ, Rinaldo-Matthis A, Wheelock CE, Wetterholm A. Advances in eicosanoid research, novel therapeutic implications. Biochem Biophys Res Commun. 2010; 396:135–139. PMID: 20494126.
Article11. Tsikas D, Zoerner AA. Analysis of eicosanoids by LC-MS/MS and GC-MS/MS: a historical retrospect and a discussion. J Chromatogr B Analyt Technol Biomed Life Sci. 2014; 964:79–88.
Article12. Tsikas D, Suchy MT. Protocols for the measurement of the F2-isoprostane, 15(S)-8-iso-prostaglandin F2α, in biological samples by GC-MS or GC-MS/MS coupled with immunoaffinity column chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2014; DOI: 10.1016/j.jchromb.2104.12.019.13. Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009; 81:8085–8093. PMID: 19715299.
Article14. Ostermann AI, Wilenberg I, Schebb NH. Comparison of sample preparation methods for the quantitative analysis of eicosanoids and other oxylipins in plasma by means of LC-MS/MS. Anal Bioanal Chem. 2015; 407:1403–1414. PMID: 25542569.
Article15. Willenberg I, Ostermann AI, Schebb NH. Targeted metabolomics of the arachidonic acid cascade: current state and challenges of LC-MS analysis of oxylipins. Anal Bioanal Chem. 2015; 407:2675–2683. PMID: 25577350.
Article16. Adcock IM, Lane SJ. Corticosteroid-insensitive asthma: molecular mechanisms. J Endocrinol. 2003; 178:347–355. PMID: 12967328.
Article17. Barnes PJ. How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol. 2006; 148:245–254. PMID: 16604091.
Article18. Loria RM, Inge TH, Cook SS, Szakal AK, Regelson W. Protection against acute lethal viral infections with the native steroid dehydroepiandrosterone (DHEA). J Med Virol. 1988; 26:301–314. PMID: 2974468.
Article19. Cutolo M. Androgens in rheumatoid arthritis: when are they effectors? Arthritis Res Ther. 2009; 11:126. PMID: 19804618.
Article20. Stárka L, Bicíková M, Hampl R. Epitestosterone - an endogenous anti-androgen? J Steroid Biochem. 1989; 33:1019–1021. PMID: 2532272.21. Bicíková M, Kanceva R, Lapcík O, Hill M, Stárka L. The effect of epitestosterone on the plasma levels of LH and FSH in ovariectomized immature rats. J Steroid Biochem Mol Biol. 1993; 44:321–324. PMID: 8499340.22. Choi MH, Skipper PL, Wishnok JS, Tannenbaum SR. Characterization of testosterone 11β-hydroxylation catalyzed by human liver microsomal cytochrome P450. Drug Metab Dispos. 2005; 33:714–718. PMID: 15764715.23. Chalbot S, Morfin R. Human liver S9 fractions: metabolism of dehydroepiandrosterone, epiandrosterone and related 7-hydroxylated derivatives. Drug Metab Dispos. 2005; 33:563–569. PMID: 15650074.
Article24. Lee SH, Lee DH, Lee J, Lee WY, Chung BC, Choi MH. Comparative GC-MS based in vitro assays of 5α-reductase activity using rat liver S9 fraction. Mass Spectrom Lett. 2012; 3:21–24.
Article25. Zhang B, Saku K. Control of matrix effects in the analysis of urinary F2-isoprostanes using novel multidimensional solid-phase extraction and LC-MS/MS. J Lipid Res. 2007; 48:733–744. PMID: 17215547.
Article26. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011; 31:986–1000. PMID: 21508345.27. Choi MH, Chung BC. Bringing GC-MS profiling of steroids into clinical applications. Mass Spectrom Rev. 2015; 34:219–236. PMID: 24965919.
Article28. Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011; 50:115–131. PMID: 20970452.
Article29. Issan Y, Hochhauser E, Guo A, Gotlinger KH, Kornowski R, Leshem-Lev D, et al. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat. 2013; 100-101:15–21. PMID: 23291334.
Article30. Erol K, Sirmagul B, Kilic FS, Yigitaslan S, Dogan AE. The role of inflammation and COX-derived prostanoids in the effects of bradykinin on isolated rat aorta and urinary bladder. Inflammation. 2012; 35:420–428. PMID: 21537904.
Article31. Maier TJ, Tausch L, Hoernig M, Coste O, Schmidt R, Angioni C, et al. Celecoxib inhibits 5-lipoxygenase. Biochem Pharmacol. 2008; 76:862–872. PMID: 18692027.
Article32. Abdel-Razzak Z, Loyer P, Fautrel A, Gautier JC, Corcos L, Turlin B, et al. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol. 1993; 44:707–715. PMID: 8232220.33. Vane JR, Botting RM. New insights into the mode of action of anti-inflammatory drugs. Inflamm Res. 1995; 44:1–10. PMID: 7664022.
Article34. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004; 89:3313–3318. PMID: 15240608.
Article35. Choi MH, Yoo YS, Chung BC. Biochemical roles of testosterone and epitestosterone to 5 alpha-reductase as indicators of male-pattern baldness. J Invest Dermatol. 2001; 116:57–61. PMID: 11168798.36. Magro CM, Rossi A, Poe J, Manhas-Bhutani S, Sadick N. The role of inflammation and immunity in the pathogenesis of androgenetic alopecia. J Drug Dermatol. 2011; 10:1404–1411.37. Yanni AE. The laboratory rabbit: an animal model of atherosclerosis research. Lab Anim. 2004; 38:246–256. PMID: 15207035.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metabolism and excretion of novel pulmonary-targeting docetaxel liposome in rabbits
- Identification of Novel Metabolic Proteins Released by Insulin Signaling of the Rat Hypothalmus Using Liquid Chromatography-Mass Spectrometry (LC-MS)
- Sporozoite proteome analysis of Cryptosporidium parvum by one-dimensional SDS-PAGE and liquid chromatography tandem mass spectrometry
- Metabolomic analysis of healthy human urine following administration of glimepiride using a liquid chromatography-tandem mass spectrometry
- Mass spectrometry based cellular phosphoinositides profiling and phospholipid analysis: A brief review