Korean J Gastroenterol.
2016 Mar;67(3):132-136. 10.4166/kjg.2016.67.3.132.
Renewed 2015 Clinical Practice Guidelines for Management of Hepatitis C by Korean Association for the Study of the Liver; What Has Been Changed? - Treatment of Chronic Hepatitis C Genotype 2 and 3
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. 93cool@hanmail.net
- KMID: 2373412
- DOI: http://doi.org/10.4166/kjg.2016.67.3.132
Abstract
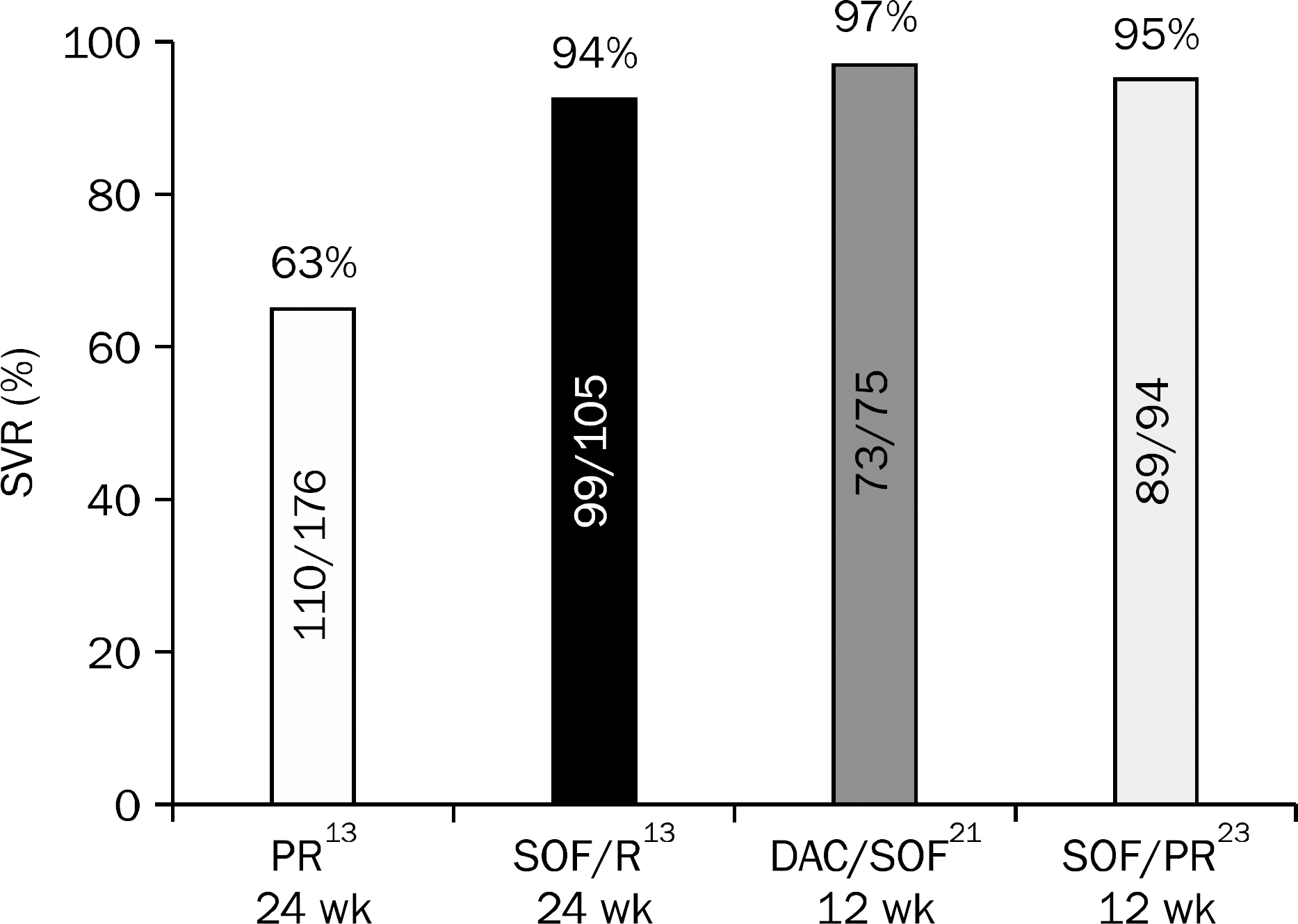
- Ever since direct-acting antiviral agents (DAA) have been approved and released into the world, numerous studies on the efficacy, adverse effects and drug-drug interactions of interferon-free DAA combination therapy have been studied and published. With all oral DAA therapy showing sustained virological response rate of 80-90% with minimal adverse events, HCV eradication has now become a realistic goal. DAA combination treatments were approved and adapted to practice in Korea in 2015, and Korean Association for the Study of the Liver (KASL) has revised the guideline based on the systematic approach that reflects evidence-based medicine and expert opinions. In this article, new recommendations for treatment of chronic HCV genotype 2 and 3 infected patients will be introduced base on KASL practice guidelines for management of hepatitis C that has been updated in 2015.
MeSH Terms
-
Antiviral Agents/*therapeutic use
Drug Therapy, Combination
Genotype
Hepacivirus/*genetics/isolation & purification
Hepatitis C/*drug therapy/virology
Humans
Interferon-alpha/therapeutic use
Practice Guidelines as Topic
Republic of Korea
Ribavirin/therapeutic use
Sofosbuvir/therapeutic use
Antiviral Agents
Interferon-alpha
Ribavirin
Sofosbuvir
Figure
Cited by 2 articles
-
Recent outbreaks of hepatitis C virus infection in Korea and strategy for prevention
In Hee Kim
J Korean Med Assoc. 2016;59(12):912-915. doi: 10.5124/jkma.2016.59.12.912.Real-world Effectiveness and Safety of Direct-acting Antiviral Agents in Patients with Chronic Hepatitis C Genotype 2 Infection: Korean Multicenter Study
Yeo Wool Kang, Yang Hyun Baek, Sung Wook Lee, Sung-Jae Park, Jun Sik Yoon, Ki Tae Yoon, Youngmi Hong, Nae-Yun Heo, Kwang Il Seo, Sang Soo Lee, Hyun Chin Cho, Jung Woo Shin
J Korean Med Sci. 2021;36(21):e142. doi: 10.3346/jkms.2021.36.e142.
Reference
-
References
1. Park SH, Park CK, Lee JW, et al. Efficacy and tolerability of peginterferon alpha plus ribavirin in the routine daily treatment of chronic hepatitis C patients in Korea: a multicenter, retrospective observational study. Gut Liver. 2012; 6:98–106.
Article2. Jin YJ, Lee JW, Lee JI, et al. Multicenter comparison of PEG-IFN α2a or α2b plus ribavirin for treatmentnaïve HCV patient in Korean population. BMC Gastroenterol. 2013; 13:74.
Article3. Shiffman ML, Suter F, Bacon BR, et al. ACCELERATE Investigators. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007; 357:124–134.
Article4. Lagging M, Langeland N, Pedersen C, et al. NORDynamIC Study Group. Randomized comparison of 12 or 24 weeks of peginterferon alpha-2a and ribavirin in chronic hepatitis C virus genotype 2/3 infection. Hepatology. 2008; 47:1837–1845.5. Hadziyannis SJ, Sette H Jr, Morgan TR, et al. PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004; 140:346–355.6. Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006; 55:1350–1359.
Article7. Foster GR, Hézode C, Bronowicki JP, et al. Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology. 2011; 141:881–889.e1.
Article8. Silva MO, Treitel M, Graham DJ, et al. Antiviral activity of boceprevir monotherapy in treatmentnaive subjects with chronic hepatitis C genotype 2/3. J Hepatol. 2013; 59:31–37.
Article9. Ludmerer SW, Graham DJ, Boots E, et al. Replication fitness and NS5B drug sensitivity of diverse hepatitis C virus isolates characterized by using a transient replication assay. Antimicrob Agents Chemother. 2005; 49:2059–2069.
Article10. Svarovskaia ES, Dvory-Sobol H, Parkin N, et al. Infrequent development of resistance in genotype 1–6 hepatitis C virus-infected subjects treated with sofosbuvir in phase 2 and 3 clinical trials. Clin Infect Dis. 2014; 59:1666–1674.
Article11. European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015; 63:199–236.12. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015; 62:932–954.13. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013; 368:1878–1887.
Article14. Kao JH, Ahn SH, Chien RN, et al. 98% SVR12 in Korean and Taiwanese patients with chronic genotype 2 HCV infection receiving 12 weeks of sofosbuvir plus ribavirin: Results from an international, multicenter phase 3 study. J Hepatol. 2015; 62:S638.15. Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013; 368:1867–1877.
Article16. Zeuzem S, Dusheiko GM, Salupere R, et al. VALENCE Investigators. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014; 370:1993–2001.
Article17. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014; 370:211–221.
Article18. Wyles DL, Ruane PJ, Sulkowski MS, et al. ALLY-2 Investigators. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015; 373:714–725.19. Foster GR, Pianko S, Brown A, et al. BOSON Study Group. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatmentexperienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology. 2015; 149:1462–1470.
Article20. Poordad F, Schiff ER, Vierling JM, et al. Daclatasvir With Sofosbuvir and Ribavirin for HCV Infection With Advanced Cirrhosis or Post-Liver Transplant Recurrence. Hepatology. 2016. [Epub ahead of print].21. Nelson DR, Cooper JN, Lalezari JP, et al. ALLY-3 Study Team. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015; 61:1127–1135.
Article22. Hezode C, De Ledinghen V, Fontaine H, et al. Daclatasvir plus sofosbuvir with or without ribavirin in patients with Hcv genotype 3 infection: interim analysis of a French multicenter compassionate use program. J Hepatol. 2015; 62:S265.23. Foster GR, Pianko S, Cooper C, et al. Sofosbuvir plus peg-IFN/RBV for 12 weeks vs sofosbuvir/RBV for 16 or 24 weeks in genotype 3 HCV-infected patients and treatmentexperienced cirrhotic patients with genotype 2 HCV: the BOSON Study. J Hepatol. 2015; 62:S259.24. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of hepatitis C. Clin Mol Hepatol. 2014; 20:89–136.25. Jung YK, Kim JH, Ahn SM, et al. Role of interleukin 28B-related gene polymorphisms in chronic hepatitis C and the response to antiviral therapy in Koreans. J Clin Gastroenterol. 2013; 47:644650.
Article26. Myers RP, Shah H, Burak KW, Cooper C, Feld JJ. An update on the management of chronic hepatitis C:2015 Consensus guidelines from the Canadian Association for the Study of the Liver. Can J Gastroenterol Hepatol. 2015; 29:19–34.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 2017 KASL clinical practice guidelines management of hepatitis C: Treatment of chronic hepatitis C
- 2017 Korean Association for the Study of the Liver (KASL) Clinical Practice Guidelines of Chronic Hepatitis C: What's New?
- 2018 Korean Association for the Study of the Liver (KASL) Clinical Practice Guidelines of Chronic Hepatitis B: What's Different?
- Renewed 2015 Clinical Practice Guidelines for Management of Hepatitis C by Korean Association for the Study of the Liver; What Has Been Changed? - Treatment of Chronic Hepatitis C Genotype 1
- KASL clinical practice guidelines for management of chronic hepatitis B