Korean J Gastroenterol.
2019 Mar;73(3):132-140. 10.4166/kjg.2019.73.3.132.
2018 Korean Association for the Study of the Liver (KASL) Clinical Practice Guidelines of Chronic Hepatitis B: What's Different?
- Affiliations
-
- 1Department of Internal Medicine, Korea University Guro Hospital, Seoul, Korea. kjhhepar@naver.com
- KMID: 2442079
- DOI: http://doi.org/10.4166/kjg.2019.73.3.132
Abstract
- The clinical practice guideline for the management of chronic hepatitis B (CHB) was originally enacted in 2004 by the Korean Association for the Study of the Liver in order to provide medical practitioners with specific medical information regarding CHB to help them facilitate their understanding of the disease and treatment of the infected patients. Other than an update on the treatment of antiviral resistance in 2014, which is a partial revision, the guidelines for the treatment of chronic hepatitis B have been revised entirely three times in 2007, 2011, and 2015. Although several major international liver association have established and revised clinical practice guidelines, since the medical environment in each country is somewhat different depending on race, region, institution, and economic conditions, it is necessary to revise the Korean guidelines to that reflect our medical environment and own research results. In this review, major change and its background will be summarized about 2018 updated clinical practice guidelines for the management of CHB.
Keyword
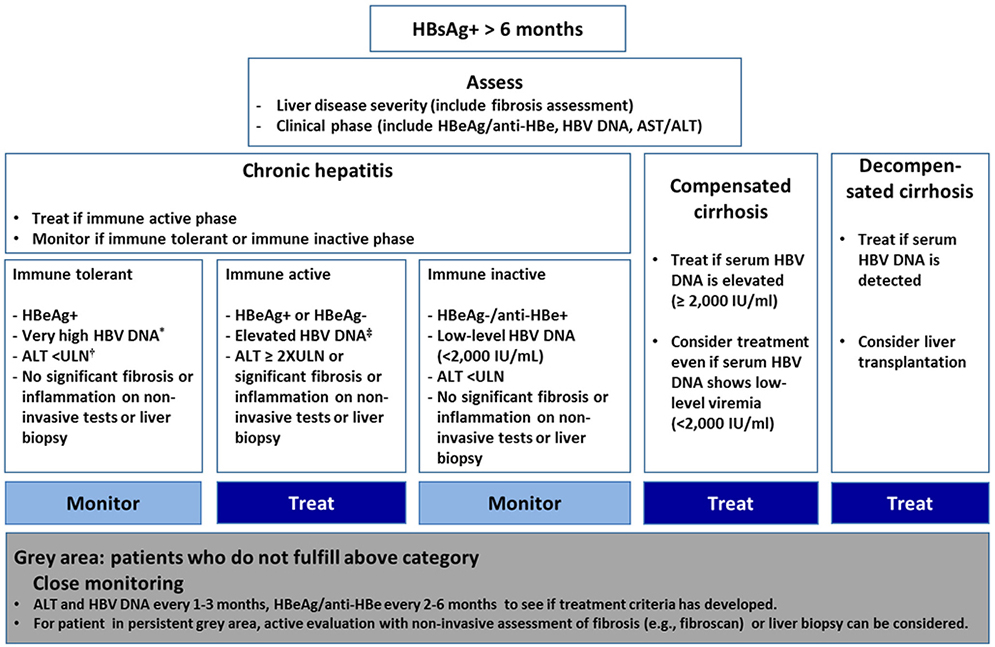
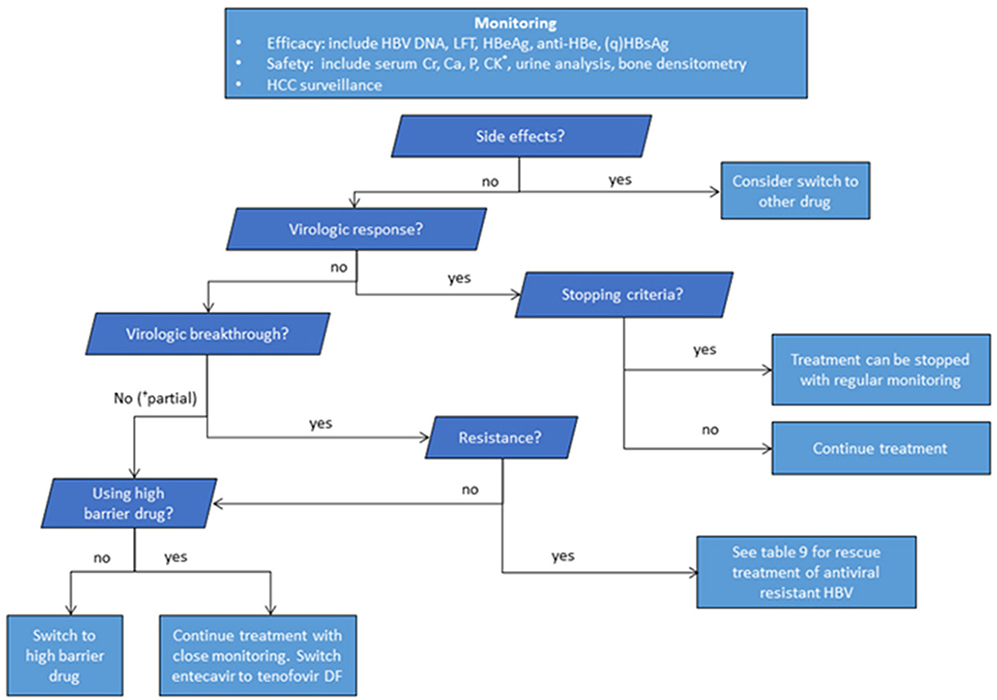
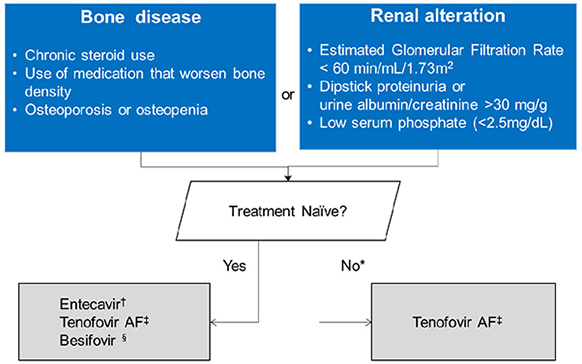
Figure
Reference
-
1. Kim NH, Cho YK, Kim BI, Kim HJ. Effect of metabolic syndrome on the clinical outcomes of chronic hepatitis B patients with nucleos(t)ide analogues treatment. Dig Dis Sci. 2018; 63:2792–2799.
Article2. Shim JJ, Kim JW, Oh CH, et al. Serum alanine aminotransferase level and liver-related mortality in patients with chronic hepatitis B: a large national cohort study. Liver Int. 2018; 38:1751–1759.
Article3. Kim GA, Lim YS, Han S, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018; 67:945–952.
Article4. Sinn DH, Lee JH, Kim K, et al. A novel model for predicting hepatocellular carcinoma development in patients with chronic hepatitis B and normal alanine aminotransferase levels. Gut Liver. 2017; 11:528–534.
Article5. Kim MN, Kim SU, Kim BK, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology. 2015; 61:1851–1859.
Article6. Chan HL, Chen YC, Gane EJ, et al. Randomized clinical trial: efficacy and safety of telbivudine and lamivudine in treatment-naïve patients with HBV-related decompensated cirrhosis. J Viral Hepat. 2012; 19:732–743.
Article7. Chang Y, Choe WH, Sinn DH, et al. Nucleos(t)ide analogue treatment for patients with hepatitis B virus (HBV) e antigen-positive chronic HBV genotype C infection: a nationwide, multicenter, retrospective study. J Infect Dis. 2017; 216:1407–1414.
Article8. Kim HS, Kim HJ, Shin WG, et al. Predictive factors for early HBeAg seroconversion in acute exacerbation of patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2009; 136:505–512.
Article9. Song BC, Cho YK, Jwa H, et al. Is it necessary to delay antiviral therapy for 3-6 months to anticipate HBeAg seroconversion in patients with HBeAg-positive chronic hepatitis B in endemic areas of HBV genotype C? Clin Mol Hepatol. 2014; 20:355–360.
Article10. de Niet A, Jansen L, Stelma F, et al. Peg-interferon plus nucleotide analogue treatment versus no treatment in patients with chronic hepatitis B with a low viral load: a randomised controlled, open-label trial. Lancet Gastroenterol Hepatol. 2017; 2:576–584.
Article11. Cho YY, Lee JH, Chang Y, et al. Comparison of overall survival between antiviral-induced viral suppression and inactive phase chronic hepatitis B patients. J Viral Hepat. 2018; 25:1161–1171.
Article12. Agarwal K, Brunetto M, Seto WK, et al. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018; 68:672–681.13. Ahn SH, Kim W, Yim HJ, et al. Continuing besifovir dipivoxil maleate versus switching from tenofovir disoproxil fumarate for treatment of chronic hepatitis B: 96 weeks results of phase 3 trial. J Hepatol. 2018; 68:Suppl 1. S87–S88.
Article14. Heo J, Park JY, Lee HJ, et al. A 96-week randomized trial of switching to entecavir in chronic hepatitis B patients with a partial virological response to lamivudine. Antivir Ther. 2012; 17:1563–1570.15. Yim HJ, Kim IH, Suh SJ, et al. Switching to tenofovir vs continuing entecavir for hepatitis B virus with partial virologic response to entecavir: a randomized controlled trial. J Viral Hepat. 2018; 25:1321–1330.
Article16. Chen J, Zhao SS, Liu XX, Huang ZB, Huang Y. Comparison of the efficacy of tenofovir versus tenofovir plus entecavir in the treatment of chronic hepatitis B in patients with poor efficacy of entecavir: a systematic review and meta-analysis. Clin Ther. 2017; 39:1870–1880.
Article17. Kim GA, Lim YS, An J, et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut. 2014; 63:1325–1332.
Article18. Kim JH, Lee YS, Lee HJ, et al. HBsAg seroclearance in chronic hepatitis B: implications for hepatocellular carcinoma. J Clin Gastroenterol. 2011; 45:64–68.19. Yip TC, Chan HL, Wong VW, Tse YK, Lam KL, Wong GL. Impact of age and gender on risk of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Hepatol. 2017; 67:902–908.
Article20. Amini-Bavil-Olyaee S, Herbers U, Sheldon J, Luedde T, Trautwein C, Tacke F. The rtA194T polymerase mutation impacts viral replication and susceptibility to tenofovir in hepatitis B e antigen-positive and hepatitis B e antigen-negative hepatitis B virus strains. Hepatology. 2009; 49:1158–1165.
Article21. Delaney WE 4th, Ray AS, Yang H, et al. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother. 2006; 50:2471–2477.
Article22. Sheldon J, Camino N, Rodés B, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther. 2005; 10:727–734.23. Lee JH, Lee YB, Cho H, et al. Identification of a triple mutation that confers tenofovir resistance in chronic hepatitis B patients. Hepatology. 2017; 66:Suppl 1. 69A–70A.24. Huang L, Li J, Yan J, et al. Antiviral therapy decreases viral reactivation in patients with hepatitis B virus-related hepatocellular carcinoma undergoing hepatectomy: a randomized controlled trial. J Viral Hepat. 2013; 20:336–342.
Article25. Dan JQ, Zhang YJ, Huang JT, et al. Hepatitis B virus reactivation after radiofrequency ablation or hepatic resection for HBV-related small hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol. 2013; 39:865–872.
Article26. Jang JW, Choi JY, Bae SH, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006; 43:233–240.
Article27. Kim JH, Park JW, Kim TH, Koh DW, Lee WJ, Kim CM. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2007; 69:813–819.
Article28. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017; 152:1297–1309.
Article29. Liu WP, Wang XP, Zheng W, et al. Hepatitis B virus reactivation after withdrawal of prophylactic antiviral therapy in patients with diffuse large B cell lymphoma. Leuk Lymphoma. 2016; 57:1355–1362.
Article30. Cerva C, Colagrossi L, Maffongelli G, et al. Persistent risk of HBV reactivation despite extensive lamivudine prophylaxis in haematopoietic stem cell transplant recipients who are anti-HBc-positive or HBV-negative recipients with an anti-HBc-positive donor. Clin Microbiol Infect. 2016; 22:946.e1–946.e8.
Article31. Huprikar S, Danziger-Isakov L, Ahn J, et al. Solid organ transplantation from hepatitis B virus-positive donors: consensus guidelines for recipient management. Am J Transplant. 2015; 15:1162–1172.
Article32. Yoo JJ, Cho EJ, Cho YY, et al. Efficacy of antiviral prophylaxis in HBsAg-negative, anti-HBc positive patients undergoing hematopoietic stem cell transplantation. Liver Int. 2015; 35:2530–2536.
Article33. Kim JH, Lee YS, Lee HS, et al. The role of tenofovir for prevention of vertical transmission of hepatitis B virus: systematic review with meta-analysis. The Editorial Office of Gut and Liver. Gut and liver: Korea digestive disease week 2018. Seoul: MEDrang Inc.;2018. p. 109.34. Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016; 374:2324–2334.
Article35. Benaboud S, Pruvost A, Coffie PA, et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d'Ivoire, in the ANRS 12109 TEmAA study, step 2. Antimicrob Agents Chemother. 2011; 55:1315–1317.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- KASL clinical practice guidelines for management of chronic hepatitis B
- 2017 KASL clinical practice guidelines management of hepatitis C: Treatment of chronic hepatitis C
- KASL clinical practice guidelines for management of chronic hepatitis B
- KASL clinical practice guidelines: management of chronic hepatitis B
- 2017 Korean Association for the Study of the Liver (KASL) Clinical Practice Guidelines of Chronic Hepatitis C: What's New?