J Gastric Cancer.
2011 Jun;11(2):122-125.
Mixed Exocrine and Endocrine Carcinoma in the Stomach: A Case Report
- Affiliations
-
- 1Division of Gastrointestinal Surgery, Department of Surgery, The Catholic University of Korea, School of Medicine, Seoul, Korea. chpark@catholic.ac.kr
- 2Department of Pathology, The Catholic University of Korea, School of Medicine, Seoul, Korea.
Abstract
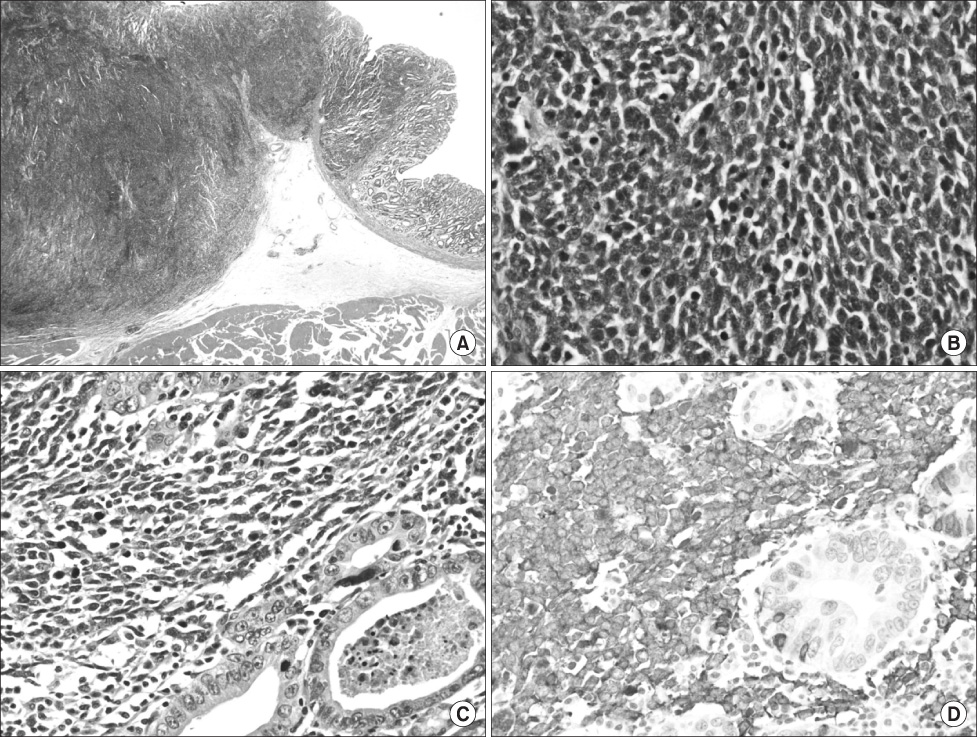
- We report a rare case of the coexistence of a gastric small cell neuroendocrine carcinoma with a gastric adenocarcinoma. A 62-year-old man presented with epigastric soreness for 1 month. Esophagogastroduodenoscopy revealed a Borrmann type I tumor at the lesser curvature of the lower body of the stomach. The patient underwent a distal gastrectomy with D2 lymph node dissection and the resected specimen exhibited a 3.5x3.5 cm sized, fungating lesion. Two separated, not intermingling, lesions with non-adenocarcinoma components encircled by well differentiated adenocarcinoma components were identified microscopically. The non-adenocarcinoma component showed neuroendocrine features, such as a solid and trabecular pattern, and the tumor cells showed a high nuclear grade with minimal cytoplasm, indistinct nucleoli, and positive response for synaptophysin, CD56. The final pathological diagnosis was a gastric mixed exocrine-endocrine carcinoma (MEEC) composed of an adenocarcinoma and small cell neuroendocrine carcinoma of the collision type.
MeSH Terms
Figure
Reference
-
1. Solcia E, Klöppel G, Sobin LH, Capella C, DeLellis RA, Heitz PU, editors. Histological Typing of Endocrine Tumours. WHO. World Health Organization. International Histological Classification of Tumours. 2000. 2nd ed. New York: Springer Verlag;56–70.2. Klöppel G, Anlauf M. Epidemiology, tumour biology and histopathological classification of neuroendocrine tumours of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2005. 19:507–517.
Article3. Kim EY, Park KC, Kwon JG. A case of double primary cancer: early gastric adenocarcinoma associated with adenocarcinoma and carcinoid. Korean J Gastroenterol. 2003. 42:533–538.4. Goteri G, Ranaldi R, Rezai B, Baccarini MG, Bearzi I. Synchronous mucosa-associated lymphoid tissue lymphoma and adenocarcinoma of the stomach. Am J Surg Pathol. 1997. 21:505–509.
Article5. Jiang SX, Mikami T, Umezawa A, Saegusa M, Kameya T, Okayasu I. Gastric large cell neuroendocrine carcinomas: a distinct clinicopathologic entity. Am J Surg Pathol. 2006. 30:945–953.
Article6. Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993. 104:994–1006.
Article7. Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006. 449:499–506.
Article8. Jain D, Eslami-Varzaneh F, Takano AM, Ayer U, Umashankar R, Muller R, et al. Composite glandular and endocrine tumors of the stomach with pancreatic acinar differentiation. Am J Surg Pathol. 2005. 29:1524–1529.
Article9. Capella C, La Rosa S, Uccella S, Billo P, Cornaggia M. Mixed endocrine-exocrine tumors of the gastrointestinal tract. Semin Diagn Pathol. 2000. 17:91–103.10. Haider K, Shahid RK, Finch D, Sami A, Ahmad I, Yadav S, et al. Extrapulmonary small cell cancer: a Canadian province's experience. Cancer. 2006. 107:2262–2269.11. Wong YN, Jack RH, Mak V, Henrik M, Davies EA. The epidemiology and survival of extrapulmonary small cell carcinoma in South East England, 1970-2004. BMC Cancer. 2009. 9:209.
Article12. Brenner B, Tang LH, Shia J, Klimstra DS, Kelsen DP. Small cell carcinomas of the gastrointestinal tract: clinicopathological features and treatment approach. Semin Oncol. 2007. 34:43–50.
Article13. Walenkamp AM, Sonke GS, Sleijfer DT. Clinical and therapeutic aspects of extrapulmonary small cell carcinoma. Cancer Treat Rev. 2009. 35:228–236.
Article14. Rothenstein J, Cleary SP, Pond GR, Dale D, Gallinger S, Moore MJ, et al. Neuroendocrine tumors of the gastrointestinal tract: a decade of experience at the Princess Margaret Hospital. Am J Clin Oncol. 2008. 31:64–70.15. Brennan SM, Gregory DL, Stillie A, Herschtal A, Mac Manus M, Ball DL. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer. 2010. 116:888–895.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Composite Neuroendocrine Carcinoma with Adenocarcinoma of the Stomach Misdiagnosed as a Giant Submucosal Tumor
- Mixed acinar-endocrine carcinoma of the pancreas: a case report
- Imaging Findings in a Case of Mixed Acinar-Endocrine Carcinoma of the Pancreas
- Mixed Ductal-Endocrine Carcinoma of the Pancreas: A Case Report
- Imaging Findings of Neuroendocrine Neoplasm in Biliary Duct with Liver Metastasis