J Korean Soc Radiol.
2017 Mar;76(3):221-228. 10.3348/jksr.2017.76.3.221.
Rapid Progression of Gliomatosis Cerebri to Secondary Glioblastoma, Factors That Affect the Progression Rate: A Case Report
- Affiliations
-
- 1Department of Radiology, Eulji University Hospital, Daejeon, Korea. midosyu@eulji.ac.kr
- 2Department of Neurosurgery, Eulji University Hospital, Daejeon, Korea.
- 3Department of Pathology, Eulji University School of Medicine, Daejeon, Korea.
- KMID: 2371690
- DOI: http://doi.org/10.3348/jksr.2017.76.3.221
Abstract
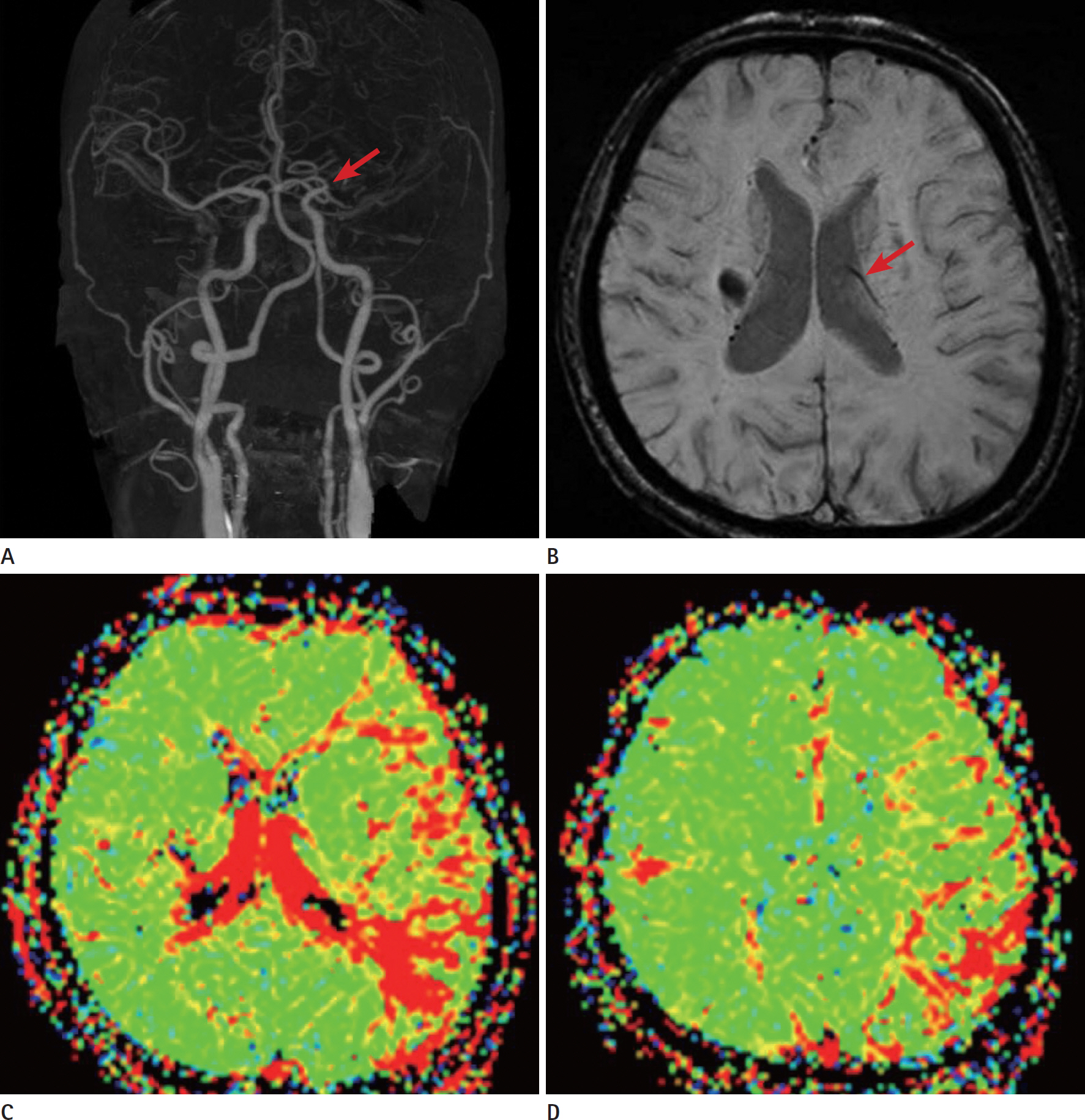
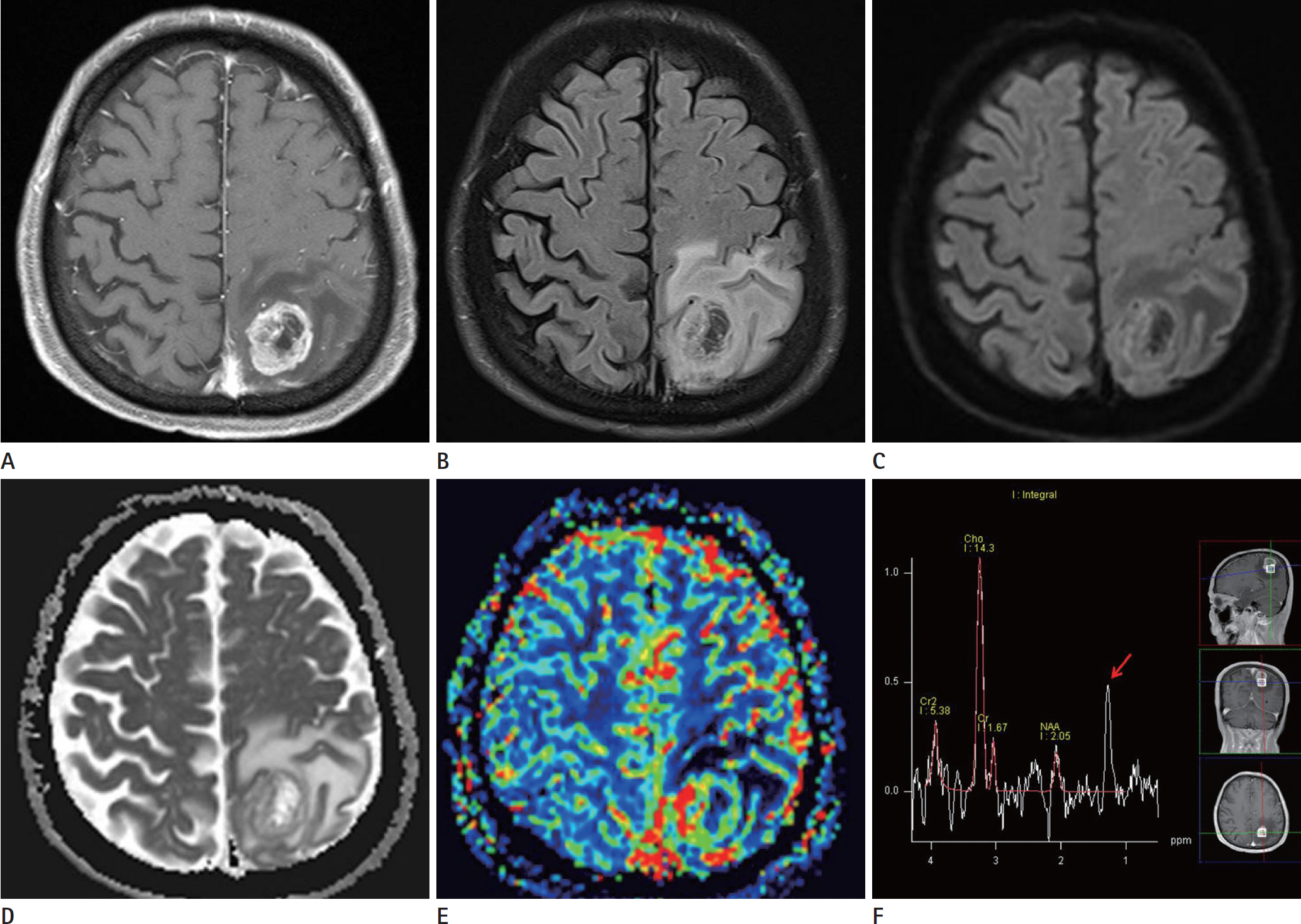
- Glioblastomas may develop de novo or through progression from low-grade or anaplastic astrocytomas. The term "˜primary glioblastoma' refers to a glioblastoma that lacks a precursor lesion and has a clinical history of less than three months. On the other hand, the term "˜secondary glioblastoma' indicates that the glioblastoma has progressed from a low-grade tumor after a long latency period and often manifests in younger patients. These subtypes of glioblastoma develop via different genetic pathways, and they differ in prognosis and response to therapy. Thus, differential diagnosis of these subtypes and prediction of the factors that affect the progression from low-grade diffuse astrocytoma to secondary glioblastoma would be clinically very important. We present a rare case of secondary glioblastoma, which developed only three months after the follow up imaging evaluations, with a history of low grade glioma, and present the factors that cause rapid progression.
MeSH Terms
Figure
Reference
-
1. Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007; 170:1445–1453.
Article2. Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996; 6:217–223. discussion 223-224.
Article3. Watanabe K, Sato K, Biernat W, Tachibana O, von Ammon K, Ogata N, et al. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biop-sies. Clin Cancer Res. 1997; 3:523–530.4. Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009; 15:6002–6007.
Article5. Scherer H. Cerebral astrocytomas and their derivatives. Am J Cancer. 1940; 40:159–198.6. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013; 19:764–772.
Article7. Burger PC, Scheithauer BW. Tumors of the central nervous system. Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology;1994.8. Kleihues P, Ohgaki H. Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro Oncol. 1999; 1:44–51.
Article9. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008; 321:1807–1812.
Article10. Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004; 9(Suppl 5):10–17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gliomatosis Cerebri in the Brain Stem and Unilateral Cerebellar Hemisphere: Case Report
- A Case of Gliomatosis Cerebri with Serial MRI Findings
- Diffusion Tensor Tractography of a Gliomatosis Cerebri: A Case Report
- Bevacizumab in Recurrent Glioma: Patterns of Treatment Failure and Implications
- A Case of Gliomatosis Cerebri; MRI and MR Spectroscopy Findings