Korean J Physiol Pharmacol.
2017 Mar;21(2):161-168. 10.4196/kjpp.2017.21.2.161.
Paracrine influence of human perivascular cells on the proliferation of adenocarcinoma alveolar epithelial cells
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Kangwon National University, Chuncheon 24341, Korea. shhong@kangwon.ac.kr medfman@gmail.com
- 2Department of Obstetrics & Gynecology, School of Medicine, Kangwon National University, Chuncheon 24341, Korea.
- 3Department of Thoracic and Cardiovascular Surgery, School of Medicine, Kangwon National University, Chuncheon 24341, Korea.
- 4Department of Molecular and Cellular Biochemistry, School of Medicine, Kangwon National University, Chuncheon 24341, Korea.
- 5Department of Medical Environmental Biology and Tropical Medicine, School of Medicine, Kangwon National University, Chuncheon 24341, Korea.
- 6Department of Physiology, School of Medicine, Kangwon National University, Chuncheon 24341, Korea.
- 7Department of Molecular Microbiology and Immunology, Department of Medicine, Alpert Medical School, Brown University, Providence, Rhode Island 02912, US.
- 8Department of Biomedical Laboratory Science, College of Health Sciences, Sanji University, Wonju 26339, Korea.
- KMID: 2371034
- DOI: http://doi.org/10.4196/kjpp.2017.21.2.161
Abstract
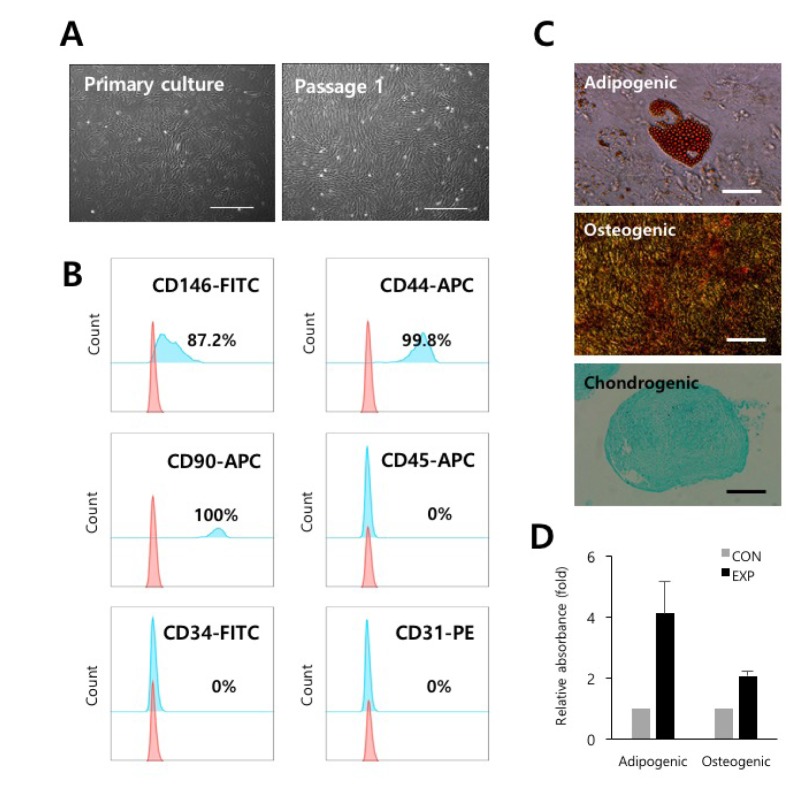
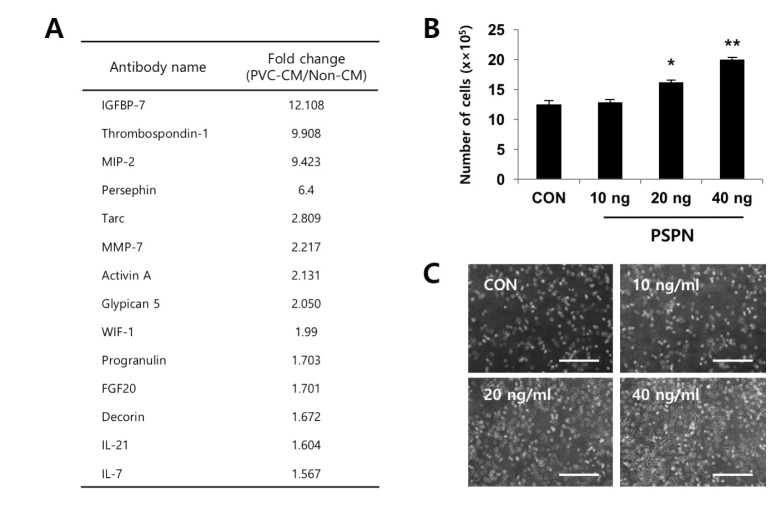
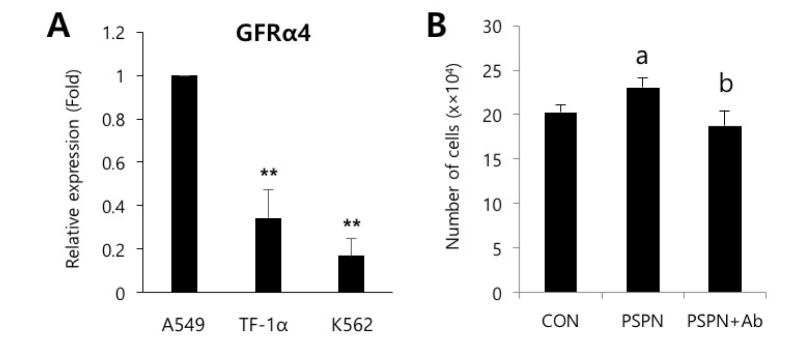
- Understanding the crosstalk mechanisms between perivascular cells (PVCs) and cancer cells might be beneficial in preventing cancer development and metastasis. In this study, we investigated the paracrine influence of PVCs derived from human umbilical cords on the proliferation of lung adenocarcinoma epithelial cells (A549) and erythroleukemia cells (TF-1α and K562) in vitro using Transwell® co-culture systems. PVCs promoted the proliferation of A549 cells without inducing morphological changes, but had no effect on the proliferation of TF-1α and K562 cells. To identify the factors secreted from PVCs, conditioned media harvested from PVC cultures were analyzed by antibody arrays. We identified a set of cytokines, including persephin (PSPN), a neurotrophic factor, and a key regulator of oral squamous cell carcinoma progression. Supplementation with PSPN significantly increased the proliferation of A549 cells. These results suggested that PVCs produced a differential effect on the proliferation of cancer cells in a cell-type dependent manner. Further, secretome analyses of PVCs and the elucidation of the molecular mechanisms could facilitate the discovery of therapeutic target(s) for lung cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Ribeiro AL, Okamoto OK. Combined effects of pericytes in the tumor microenvironment. Stem Cells Int. 2015; DOI: 10.1155/2015/868475.
Article2. Hall AP. Review of the pericyte during angiogenesis and its role in cancer and diabetic retinopathy. Toxicol Pathol. 2006; 34:763–775. PMID: 17162534.
Article3. Hay E. Unusual presentation of pheochromocytoma. Am J Emerg Med. 1991; 9:399–402. PMID: 2054015.
Article4. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008; 3:301–313. PMID: 18786417.
Article5. Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007; 25:1384–1392. PMID: 17332507.
Article6. Zebardast N, Lickorish D, Davies JE. Human umbilical cord perivascular cells (HUCPVC): A mesenchymal cell source for dermal wound healing. Organogenesis. 2010; 6:197–203. PMID: 21220956.7. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009; 10:165–177. PMID: 19234476.
Article8. Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005; 118:771–780. PMID: 15687104.
Article9. Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009; 12:125–137. PMID: 19449109.
Article10. Caporali A, Meloni M, Nailor A, Mitić T, Shantikumar S, Riu F, Sala-Newby GB, Rose L, Besnier M, Katare R, Voellenkle C, Verkade P, Martelli F, Madeddu P, Emanueli C. p75(NTR)-dependent activation of NF-κB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia. Nat Commun. 2015; 6:8024. PMID: 26268439.
Article11. An B, Na S, Lee S, Kim WJ, Yang SR, Woo HM, Kook S, Hong Y, Song H, Hong SH. Non-enzymatic isolation followed by supplementation of basic fibroblast growth factor improves proliferation, clonogenic capacity and SSEA-4 expression of perivascular cells from human umbilical cord. Cell Tissue Res. 2015; 359:767–777. PMID: 25501896.
Article12. Hong SH, Maghen L, Kenigsberg S, Teichert AM, Rammeloo AW, Shlush E, Szaraz P, Pereira S, Lulat A, Xiao R, Yie SM, Gauthier-Fisher A, Librach CL. Ontogeny of human umbilical cord perivascular cells: molecular and fate potential changes during gestation. Stem Cells Dev. 2013; 22:2425–2439. PMID: 23557155.
Article13. An B, Heo HR, Lee S, Park JA, Kim KS, Yang J, Hong SH. Supplementation of growth differentiation factor-5 increases proliferation and size of chondrogenic pellets of human umbilical cord-derived perivascular stem cells. Tissue Eng Regen Med. 2015; 12:181–187.
Article14. Kashyap MK. Role of insulin-like growth factor-binding proteins in the pathophysiology and tumorigenesis of gastroesophageal cancers. Tumour Biol. 2015; 36:8247–8257. PMID: 26369544.
Article15. Sid B, Sartelet H, Bellon G, El Btaouri H, Rath G, Delorme N, Haye B, Martiny L. Thrombospondin 1: a multifunctional protein implicated in the regulation of tumor growth. Crit Rev Oncol Hematol. 2004; 49:245–258. PMID: 15036264.
Article16. Baba T, Sakamoto Y, Kasamatsu A, Minakawa Y, Yokota S, Higo M, Yokoe H, Ogawara K, Shiiba M, Tanzawa H, Uzawa K. Persephin: A potential key component in human oral cancer progression through the RET receptor tyrosine kinase-mitogen-activated protein kinase signaling pathway. Mol Carcinog. 2015; 54:608–617. PMID: 24375483.
Article17. Wijayarathna R, de Kretser DM. Activins in reproductive biology and beyond. Hum Reprod Update. 2016; 22.
Article18. Huang Y, Du Q, Wu W, She F, Chen Y. Rescued expression of WIF-1 in gallbladder cancer inhibits tumor growth and induces tumor cell apoptosis with altered expression of proteins. Mol Med Rep. 2016; 14:2573–2581. PMID: 27430608.
Article19. Koga C, Adati N, Nakata K, Mikoshiba K, Furuhata Y, Sato S, Tei H, Sakaki Y, Kurokawa T, Shiokawa K, Yokoyama KK. Characterization of a novel member of the FGF family, XFGF-20, in Xenopus laevis. Biochem Biophys Res Commun. 1999; 261:756–765. PMID: 10441498.
Article20. Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997; 1333:F179–F199. PMID: 9426203.
Article21. Chen LH, Liu DW, Chang JL, Chen PR, Hsu LP, Lin HY, Chou YF, Lee CF, Yang MC, Wen YH, Hsu WL, Weng CF. Methylation status of insulin-like growth factor-binding protein 7 concurs with the malignance of oral tongue cancer. J Exp Clin Cancer Res. 2015; 34:20. PMID: 25880247.
Article22. Verhagen HJ, de Leeuw DC, Roemer MG, Denkers F, Pouwels W, Rutten A, Celie PH, Ossenkoppele GJ, Schuurhuis GJ, Smit L. IGFBP7 induces apoptosis of acute myeloid leukemia cells and synergizes with chemotherapy in suppression of leukemia cell survival. Cell Death Dis. 2014; 5:e1300. PMID: 24967962.
Article23. Teraoku H, Morine Y, Ikemoto T, Saito Y, Yamada S, Yoshikawa M, Takasu C, Higashijima J, Imura S, Shimada M. Role of thrombospondin-1 expression in colorectal liver metastasis and its molecular mechanism. J Hepatobiliary Pancreat Sci. 2016; 23:565–573. PMID: 27404020.
Article24. Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG, Lampe PA, Heuckeroth RO, Kotzbauer PT, Simburger KS, Golden JP, Davies JA, Vejsada R, Kato AC, Hynes M, Sherman D, Nishimura M, Wang LC, Vandlen R, Moffat B, Klein RD, Poulsen K, Gray C, Garces A, Johnson EM Jr. Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron. 1998; 20:245–253. PMID: 9491986.
Article25. Zihlmann KB, Ducray AD, Schaller B, Huber AW, Krebs SH, Andres RH, Seiler RW, Meyer M, Widmer HR. The GDNF family members neurturin, artemin and persephin promote the morphological differentiation of cultured ventral mesencephalic dopaminergic neurons. Brain Res Bull. 2005; 68:42–53. PMID: 16325003.
Article26. Roussa E, Oehlke O, Rahhal B, Heermann S, Heidrich S, Wiehle M, Krieglstein K. Transforming growth factor beta cooperates with persephin for dopaminergic phenotype induction. Stem Cells. 2008; 26:1683–1694. PMID: 18420832.27. Murata T, Tsuboi M, Koide N, Hikita K, Kohno S, Kaneda N. Neuronal differentiation elicited by glial cell line-derived neurotrophic factor and ciliary neurotrophic factor in adrenal chromaffin cell line tsAM5D immortalized with temperature-sensitive SV40 T-antigen. J Neurosci Res. 2008; 86:1694–1710. PMID: 18293415.
Article28. Yagi H, Kitagawa Y. The role of mesenchymal stem cells in cancer development. Front Genet. 2013; 4:261. PMID: 24348516.
Article29. Sinha D, Chong L, George J, Schlüter H, Mönchgesang S, Mills S, Li J, Parish C, Bowtell D, Kaur P. Australian Ovarian Cancer Study Group. Pericytes promote malignant ovarian cancer progression in mice and predict poor prognosis in serous ovarian cancer patients. Clin Cancer Res. 2016; 22:1813–1824. PMID: 26589433.
Article30. Driscoll KE. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res. 1994; 20:473–490. PMID: 7882902.
Article31. Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997; 272:15036–15042. PMID: 9169480.
Article32. Li F, Shi W, Capurro M, Filmus J. Glypican-5 stimulates rhabdomyosarcoma cell proliferation by activating Hedgehog signaling. J Cell Biol. 2011; 192:691–704. PMID: 21339334.
Article33. Nicoletto BB, Canani LH. The role of progranulin in diabetes and kidney disease. Diabetol Metab Syndr. 2015; 7:117. PMID: 26697121.
Article34. Järveläinen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biol. 2015; 43:15–26. PMID: 25661523.
Article35. Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, Schaefer L, Torres A, Iozzo RV. Decorin causes autophagy in endothelial cells via Peg3. Proc Natl Acad Sci U S A. 2013; 110:E2582–E2591. PMID: 23798385.
Article36. Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000; 408:57–63. PMID: 11081504.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Immunohistochemical Study of the Relationships between Estrogen and Progesterone Receptors and Proliferating Cell Nuclear Antigen in Endometrial Hyperplasia and Adenocarcinoma
- Inhibitory Effect of Tranilast on the Proliferation of Human Conjunctival Epithelial Cells and Subconjunctival Fibroblasts
- Culture of Retinal Pigment Epithelial Cells on Collagen Membrane
- Effect of endothelin-1 on proliferation and differentiation of rat tracheal epithelial cells
- An Experimental Study of the Effect of Cis-dichlorodiammineplatinum(II) on the Radiation-induced Lung Damage in Rat