J Korean Ophthalmol Soc.
2017 Feb;58(2):156-164. 10.3341/jkos.2017.58.2.156.
Clinical Manifestation and Outcomes of Neuroretinitis in Korea
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea. oph97@naver.com
- 2Department of Ophthalmology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea.
- KMID: 2369462
- DOI: http://doi.org/10.3341/jkos.2017.58.2.156
Abstract
- PURPOSE
In the present study, the clinical characteristics and outcomes of neuroretinitis in Korea were investigated.
METHODS
Seven consecutive patients with neuroretinitis from 2012 to 2015 were retrospectively reviewed.
RESULTS
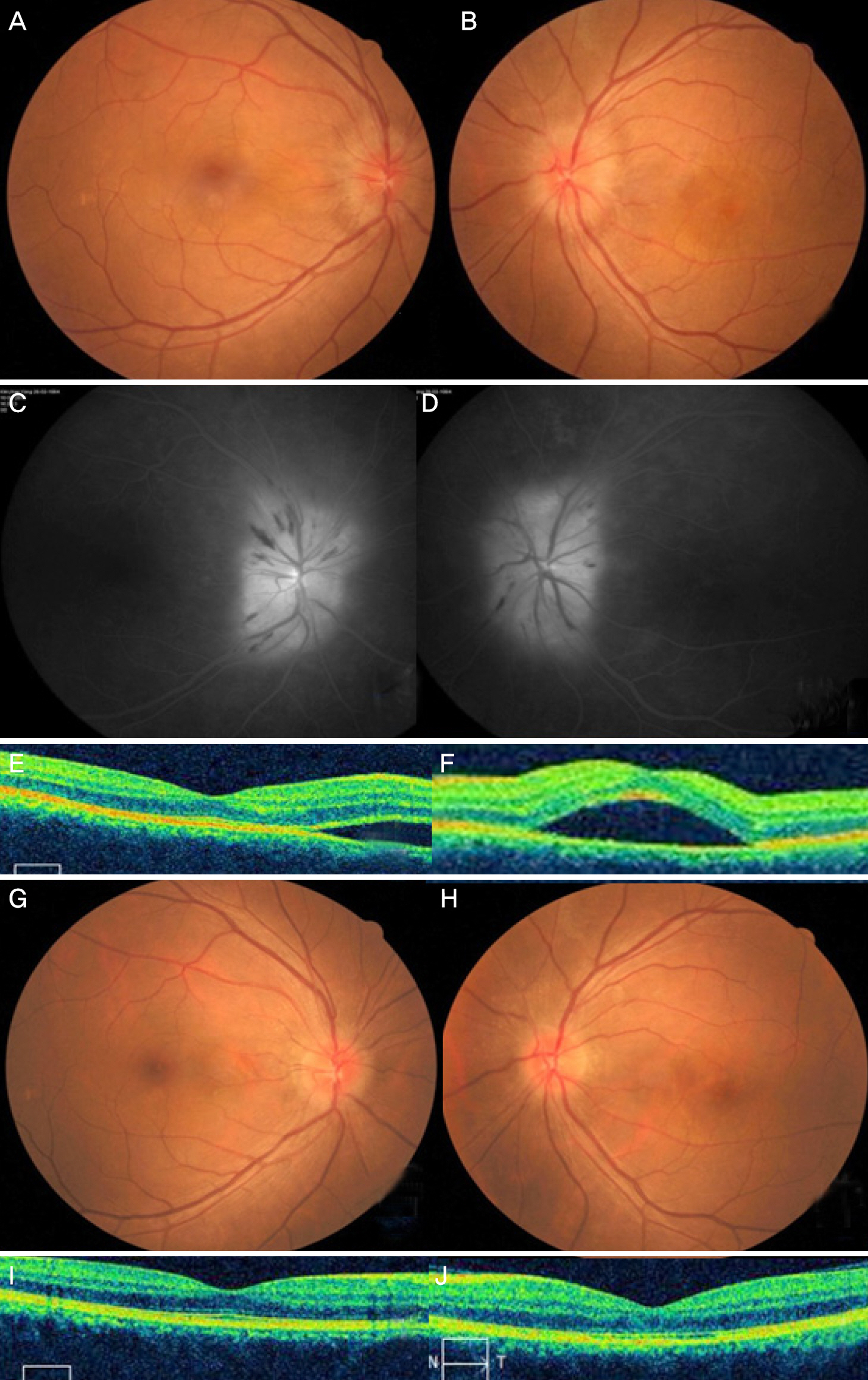
The present study included 9 eyes of 7 patients. The subjects consisted of 5 males and 2 females and the mean age was 45.1 ± 13.2 years. Two patients had Influenza-like symptoms and the others had no specific general symptoms. The mean best corrected visual acuity was logMAR 0.48 ± 0.55 (0-1.6) on the initial visit. Four patients 40 years of age or under had no abnormal findings in laboratory work-up. Conversely, 3 patients over 40 years of age were positive for Toxocara based on enzyme-linked immunosorbent assay (ELISA). Two of 3 patients positive for Toxocara were treated with albendazole and 1was not. Eight eyes had good visual recovery over 20/30 except for 1 patient who did not take the albendazole despite being positive for Toxocara detected using ELISA.
CONCLUSIONS
Three of 7 patients with neuroretinitis in Korea showed positive results for Toxocara based on ELISA. In cases with seropositive Toxocara ELISA results, albendazole treatment should be considered.
MeSH Terms
Figure
Reference
-
References
1. Purvin V, Sundaram S, Kawasaki A. Neuroretinitis: review of the literature and new observations. J Neuroophthalmol. 2011; 31:58–68.
Article2. Stewart MW, Brazis PW, Barrett KM. . Optical coherence to-mography in a case of bilateral neuroretinitis. J Neuroophthalmol. 2005; 25:131–3.
Article3. Suhler EB, Lauer AK, Rosenbaum JT. Prevalence of serologic evi-dence of cat scratch disease in patients with neuroretinitis. Ophthalmology. 2000; 107:871–6.4. Arruga J, Valentines J, Mauri F. . Neuroretinitis in acquired syphilis. Ophthalmology. 1985; 92:262–70.
Article5. Folk JC, Weingeist TA, Corbett JJ. . Syphilitic. neuroretinitis. Am J Ophthalmol. 1983; 95:480–6.
Article6. Ninomiya H, Hamada T, Akiya S, Kazama H. Three cases of acute syphilitic neuroretinitis. Folia Ophthalmologica Japonica. 1990; 41:2088–92.7. Karma A, Stenborg T, Summanen P. . Long-term follow-up of chronic Lyme neuroretinitis. Retina. 1996; 16:505–9.
Article8. Dreyer RF, Hopen G, Gass JD, Smith JL. Leber's idiopathic stellate neuroretinitis. Arch Ophthalmol. 1984; 102:1140–5.
Article9. Perrotta S, Nobili B, Grassia C. . Bilateral neuroretinitis in a 6-year-old boy with acquired toxoplasmosis. Arch Ophthalmol. 2003; 121:1493–6.
Article10. Küçükerdönmez C, Akova YA, Yilmaz G.Ocular toxoplasmosis presenting as neuroretinitis: report of two cases. Ocul Immunol Inflamm. 2002; 10:229–34.
Article11. Stechschulte SU, Kim RY, Cunningham ET Jr. Tuberculous neuroretinitis. J Neuroophthalmol. 1999; 19:201–4.
Article12. Bird AC, Smith JL, Curtin VT. Nematode optic neuritis. Am J Ophthalmol. 1970; 69:72–7.
Article13. Kim MH, Kim BN, Han TH. Cat-scratch disease: a case report and literature review of human and animal studies performed in Korea. Infect Chemother. 2012; 44:299–302.
Article14. Yang HK, Woo SJ, Hwang JM. Toxocara optic neuropathy after in-gestion of raw meat products. Optom Vis Sci. 2014; 91:e267–73.
Article15. Jee D, Kim KS, Lee WK. . Clinical features of ocular toxocar-iasis in adult Korean patients. Ocul Immunol Inflamm. 2016; 24:207–16.
Article16. Lee DH, Koh HJ, Kim SS. . Intravitreal bevacizumab injection for serous retinal detachment associated with Leber’s idiopathic stellate neuroretinitis. J Korean Ophthalmol Soc. 2014; 55:1562–6.
Article17. Leber T. The pseudoneuroretinitic retinal diseases, the retinitis stellate: retinal disease after severe damage. Graefe-Saemisch Handbook of the Complete Ophthalmology. 2nd ed. Leipzig: Engelmann;1916. p. 1319.18. Gass JD. Diseases of the optic nerve that may simulate macular disease. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977; 83:763.19. Ando R, Shinmei Y, Nitta T. . Central serous retinal detach-ment detected by optical coherence tomography in Leber's idio-pathic stellate neuroretinitis. Jpn J Ophthalmol. 2005; 49:547–8.
Article20. Kitamei H, Suzuki Y, Takahashi M. . Retinal angiography and optical coherence tomography disclose focal optic disc vascular leakage and lipid-rich fluid accumulation within the retina in a pa-tient with Leber idiopathic stellate neuroretinitis. J Neuroophthalmol. 2009; 29:203–7.
Article21. Wolter JR, Phillips RL, Butler RG. The star-figure of the macular area; histopathological study of a case of angiospatic (hypertensive) retinopathy. AMA Arch Ophthalmol. 1958; 60:49–59.22. Kaliaperumal S, Narayan S. Neuroretinitis: update on a visual emergency and role of technology in its diagnosis. J Biomed Sci Eng. 2013; 6:15.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unilateral neuroretinitis and periparillary serous retinal detachment in cat-scratch disease
- A Case of Tubeculous Neuroretinitis
- Ocular Manifestations Associated with Cerebral Vein Sinus Thrombosis Developing after SARS-CoV-2 Vaccination
- Diffuse Unilateral Subacute Neuroretinitis in a Healthy Korean Male: The First Case Report in Korea
- Intravitreal Bevacizumab Injection for Serous Retinal Detachment Associated with Leber's Idiopathic Stellate Neuroretinitis