Immune Netw.
2017 Feb;17(1):13-19. 10.4110/in.2017.17.1.13.
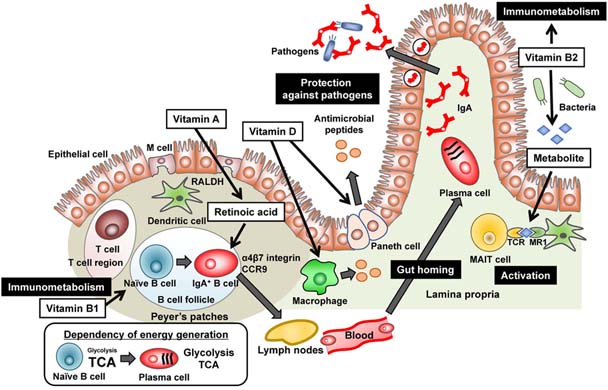
The Specific Roles of Vitamins in the Regulation of Immunosurveillance and Maintenance of Immunologic Homeostasis in the Gut
- Affiliations
-
- 1Laboratory of Vaccine Materials, National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN), Osaka 567-0085, Japan. kunisawa@nibiohn.go.jp
- 2Graduate School of Medicine, Graduate School of Pharmaceutical Sciences, Graduate School of Dentistry, Osaka University, Osaka 565-0871, Japan.
- 3Division of Mucosal Immunology, Department of Microbiology and Immunology and International Research and Development Center for Mucosal Vaccines, The Institute of Medical Science, The University of Tokyo, Tokyo 108-8639, Japan.
- 4Department of Microbiology and Immunology, Kobe University Graduate School of Medicine, Hyogo 650-0017, Japan.
- KMID: 2368972
- DOI: http://doi.org/10.4110/in.2017.17.1.13
Abstract
- Vitamins are micronutrients which are essential for the maintenance of biological responses including immune system. Hence, vitamin deficiency increases a risk of infectious, allergic, and inflammatory diseases. Accumulating evidence has recently revealed the molecular and cellular mechanisms of vitamin-mediated regulation in the active and quiescent immune responses. In this review, we focus on the immunologic roles of vitamins in the regulation of homeostasis and surveillance in the gut.
Keyword
MeSH Terms
Figure
Reference
-
1. Kunisawa J, Kiyono H. Immune regulation and monitoring at the epithelial surface of the intestine. Drug Discov Today. 2013; 18:87–92.
Article2. Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010; 10:159–169.
Article3. Brandtzaeg P. Secretory IgA: Designed for Anti-Microbial. Defense Front Immunol. 2013; 4:222.4. Kunisawa J, Kurashima Y, Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev. 2012; 64:523–530.
Article5. Nagatake T, Kunisawa J. Unique functions of mucosa-associated lymphoid tissues as targets of mucosal vaccines. Curr Topics Pharmacol. 2013; 17:13–23.6. Corthay A. How do regulatory T cells work? Scand J Immunol. 2009; 70:326–336.
Article7. Tanoue T, Atarashi K, Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol. 2016; 16:295–309.
Article8. Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014; 260:102–117.
Article9. Kotlyar DS, Shum M, Hsieh J, Blonski W, Greenwald DA. Non-pulmonary allergic diseases and inflammatory bowel disease: a qualitative review. World J Gastroenterol. 2014; 20:11023–11032.
Article10. Lamichhane A, Kiyono H, Kunisawa J. Nutritional components regulate the gut immune system and its association with intestinal immune disease development. J Gastroenterol Hepatol. 2013; 28:Suppl 4. 18–24.
Article11. Suzuki H, Kunisawa J. Vitamin-mediated immune regulation in the development of inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2015; 15:212–215.
Article12. Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014; 40:315–327.
Article13. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011; 474:327–336.
Article14. Coombes JL, Siddiqui KR, rancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007; 204:1757–1764.
Article15. Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007; 204:1775–1785.
Article16. Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007; 317:256–260.
Article17. Ouyang X, Zhang R, Yang J, Li Q, Qin L, Zhu C, Liu J, Ning H, Shin MS, Gupta M, Qi CF, He JC, Lira SA, Morse HC 3rd, Ozato K, Mayer L, Xiong H. Transcription factor IRF8 directs a silencing programme for TH17 cell differentiation. Nat Commun. 2011; 2:314.
Article18. Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008; 205:2139–2149.
Article19. Yamaguchi T, Hirota K, Nagahama K, Ohkawa K, Takahashi T, Nomura T, Sakaguchi S. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007; 27:145–159.
Article20. Kunisawa J, Hashimoto E, Ishikawa I, Kiyono H. A pivotal role of vitamin B9 in the maintenance of regulatory T cells in vitro and in vivo. PLoS One. 2012; 7:e32094.21. Kinoshita M, Kayama H, Kusu T, Yamaguchi T, Kunisawa J, Kiyono H, Sakaguchi S, Takeda K. Dietary folic acid promotes survival of Foxp3+ regulatory T cells in the colon. J Immunol. 2012; 189:2869–2878.
Article22. Grant WB, Holick MF. Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev. 2005; 10:94–111.23. Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009; 4:1151–1165.
Article24. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006; 311:1770–1773.
Article25. Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008; 19:1912–1921.
Article26. Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009; 183:5458–5467.
Article27. Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000; 164:2405–2411.
Article28. Meckel K, Li YC, Lim J, Kocherginsky M, Weber C, Almoghrabi A, Chen X, Kaboff A, Sadiq F, Hanauer SB, Cohen RD, Kwon J, Rubin DT, Hanan I, Sakuraba A, Yen E, Bissonnette M, Pekow J. Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis. Am J Clin Nutr. 2016; 104:113–120.
Article29. Kim MJ, Kim SN, Lee YW, Choe YB, Ahn KJ. Vitamin D status and efficacy of vitamin D supplementation in atopic dermatitis: A systematic review and meta-analysis. Nutrients. 2016; 8:E789.
Article30. Peng C, Wang X, Chen J, Jiao R, Wang L, Li YM, Zuo Y, Liu Y, Lei L, Ma KY, Huang Y, Chen ZY. Biology of ageing and role of dietary antioxidants. Biomed Res Int. 2014; 2014:831841.
Article31. Cook-Mills JM. Isoforms of vitamin E differentially regulate PKC alpha and inflammation: A review. J Clin Cell Immunol. 2013; 4:1000137.32. Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills JM. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009; 182:4395–4405.
Article33. Soriano A, Salas A, Salas A, Sans M, Gironella M, Elena M, Anderson DC, Pique JM, Panes J. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab Invest. 2000; 80:1541–1551.
Article34. Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E isoforms differentially regulate intercellular adhesion molecule-1 activation of PKCα in human microvascular endothelial cells. PLoS One. 2012; 7:e41054.
Article35. Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res. 2005; 49:7–30.
Article36. Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol. 2009; 21:8–13.
Article37. Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004; 21:527–538.
Article38. Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006; 314:1157–1160.
Article39. Bhaskaram P. Micronutrient malnutrition, infection, and immunity: an overview. Nutr Rev. 2002; 60:S40–S45.
Article40. Fisker AB, Bale C, Jorgensen MJ, Balde I, Hornshoj L, Bibby BM, Aaby P, Benn CS. High-dose vitamin A supplementation administered with vaccinations after 6 months of age: sex-differential adverse reactions and morbidity. Vaccine. 2013; 31:3191–3198.
Article41. Huskisson E, Maggini S, Ruf M. The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res. 2007; 35:277–289.
Article42. Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013; 38:633–643.
Article43. Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la SH, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999; 189:1907–1921.
Article44. Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, Williamson NA, Strugnell RA, Van SD, Mak JY, Fairlie DP, Kjer-Nielsen L, Rossjohn J, McCluskey J. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014; 509:361–365.
Article45. Cowley SC. MAIT cells and pathogen defense. Cell Mol Life Sci. 2014; 71:4831–4840.
Article46. Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, Chatelain D, Barre A, Nguyen-Khac E, Lantz O, Dupas JL, Treiner E. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014; 176:266–274.
Article47. Webb ME, Marquet A, Mendel RR, Rebeille F, Smith AG. Elucidating biosynthetic pathways for vitamins and cofactors. Nat Prod Rep. 2007; 24:988–1008.
Article48. Frank RA, Leeper FJ, Luisi BF. Structure, mechanism and catalytic duality of thiamine-dependent enzymes. Cell Mol Life Sci. 2007; 64:892–905.
Article49. Manzetti S, Zhang J, van der SD. Thiamin function, metabolism, uptake, and transport. Biochemistry. 2014; 53:821–835.
Article50. Kunisawa J, Sugiura Y, Wake T, Nagatake T, Suzuki H, Nagasawa R, Shikata S, Honda K, Hashimoto E, Suzuki Y, Setou M, Suematsu M, Kiyono H. Mode of bioenergetic metabolism during B cell differentiation in the intestine determines the distinct requirement for vitamin B1. Cell Rep. 2015; 13:122–131.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Roles of intestinal epithelial cells in the maintenance of gut homeostasis
- New era for mucosal mast cells: their roles in inflammation, allergic immune responses and adjuvant development
- Gut Microbiota in Graft-versus-Host Disease
- Gut Microbial Metabolites on Host Immune Responses in Health and Disease
- Vitamin D and Immune Responses