Diabetes Metab J.
2016 Aug;40(4):308-317. 10.4093/dmj.2016.40.4.308.
Application of the Oral Minimal Model to Korean Subjects with Normal Glucose Tolerance and Type 2 Diabetes Mellitus
- Affiliations
-
- 1Department of Biomedical Engineering, Seoul National University College of Medicine, Seoul, Korea. sungwan@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. ymchomd@snu.ac.kr
- 3Interdisciplinary Program of Bioengineering, Seoul National University College of Engineering, Seoul, Korea.
- KMID: 2368518
- DOI: http://doi.org/10.4093/dmj.2016.40.4.308
Abstract
- BACKGROUND
The oral minimal model is a simple, useful tool for the assessment of β-cell function and insulin sensitivity across the spectrum of glucose tolerance, including normal glucose tolerance (NGT), prediabetes, and type 2 diabetes mellitus (T2DM) in humans.
METHODS
Plasma glucose, insulin, and C-peptide levels were measured during a 180-minute, 75-g oral glucose tolerance test in 24 Korean subjects with NGT (n=10) and T2DM (n=14). The parameters in the computational model were estimated, and the indexes for insulin sensitivity and β-cell function were compared between the NGT and T2DM groups.
RESULTS
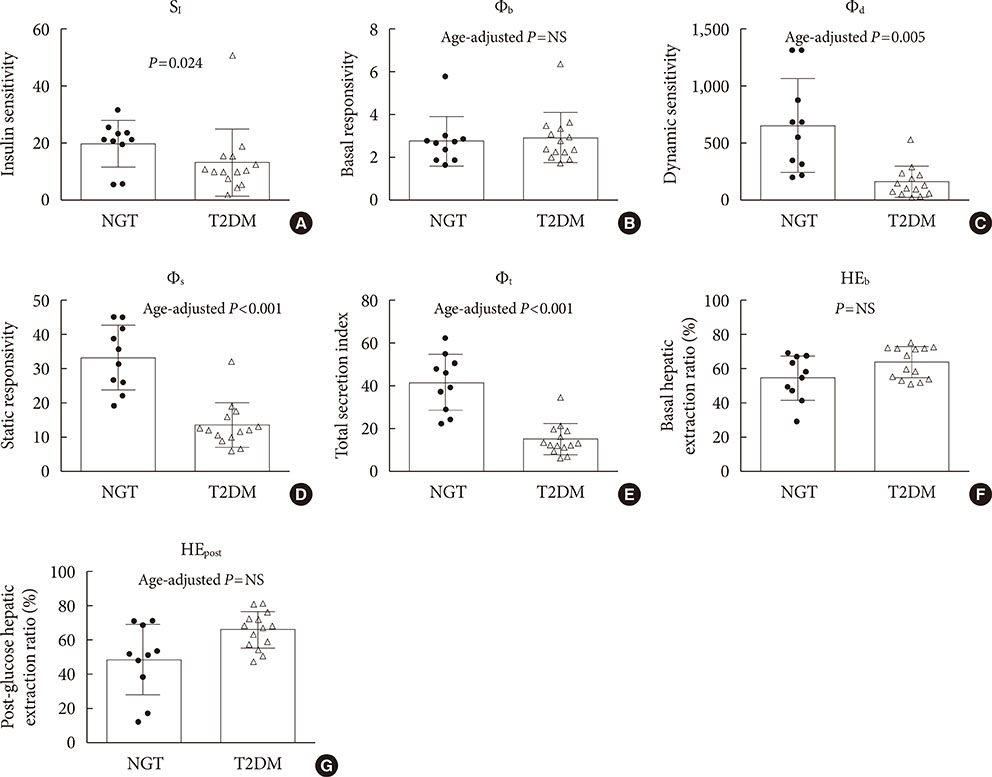
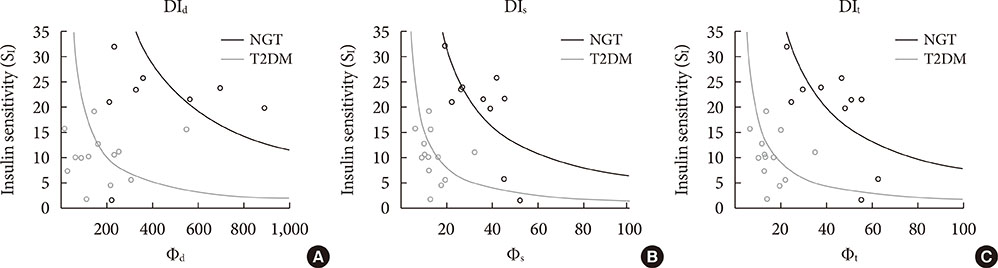
The insulin sensitivity index was lower in the T2DM group than the NGT group. The basal index of β-cell responsivity, basal hepatic insulin extraction ratio, and post-glucose challenge hepatic insulin extraction ratio were not different between the NGT and T2DM groups. The dynamic, static, and total β-cell responsivity indexes were significantly lower in the T2DM group than the NGT group. The dynamic, static, and total disposition indexes were also significantly lower in the T2DM group than the NGT group.
CONCLUSION
The oral minimal model can be reproducibly applied to evaluate β-cell function and insulin sensitivity in Koreans.
Keyword
MeSH Terms
Figure
Reference
-
1. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009; 58:773–795.2. Brubaker PL, Ohayon EL, D'Alessandro LM, Norwich KH. A mathematical model of the oral glucose tolerance test illustrating the effects of the incretins. Ann Biomed Eng. 2007; 35:1286–1300.3. Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes. 2014; 63:1203–1213.4. Cobelli C, Toffolo GM, Dalla Man C, Campioni M, Denti P, Caumo A, Butler P, Rizza R. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007; 293:E1–E15.5. Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Orsini Federici M, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004; 25:905–920.6. Lee JC, Kim M, Choi KR, Oh TJ, Kim MY, Cho YM, Kim K, Kim HC, Kim S. In silico evaluation of glucose control protocols for critically ill patients. IEEE Trans Biomed Eng. 2012; 59:54–57.7. James G, Witten D, Hastie T, Tibshirani R. An introduction to statistical learning: with applications in R. New York: Springer;2013. p. 37.8. Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979; 236:E667–E677.9. Staten MA, Kelley DE. Using oral challenge testing to assess insulin action and secretion with mathematical modeling. Diabetes. 2014; 63:1188–1190.10. Caumo A, Vicini P, Zachwieja JJ, Avogaro A, Yarasheski K, Bier DM, Cobelli C. Undermodeling affects minimal model indexes: insights from a two-compartment model. Am J Physiol. 1999; 276(6 Pt 1):E1171–E1193.11. Toffolo G, De Grandi F, Cobelli C. Estimation of beta-cell sensitivity from intravenous glucose tolerance test C-peptide data. Knowledge of the kinetics avoids errors in modeling the secretion. Diabetes. 1995; 44:845–854.12. Campioni M, Toffolo G, Basu R, Rizza RA, Cobelli C. Minimal model assessment of hepatic insulin extraction during an oral test from standard insulin kinetic parameters. Am J Physiol Endocrinol Metab. 2009; 297:E941–E948.13. Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, Cobelli C, Rizza RA. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. 2006; 55:2001–2014.14. Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 2001; 50:150–158.15. Burattini R, Morettini M. Identification of an integrated mathematical model of standard oral glucose tolerance test for characterization of insulin potentiation in health. Comput Methods Programs Biomed. 2012; 107:248–261.16. Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig. 2015; 6:495–507.17. Dalla Man C, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng. 2002; 49:419–429.18. McDonald C, Dunaif A, Finegood DT. Minimal-model estimates of insulin sensitivity are insensitive to errors in glucose effectiveness. J Clin Endocrinol Metab. 2000; 85:2504–2508.19. Caumo A, Bergman RN, Cobelli C. Insulin sensitivity from meal tolerance tests in normal subjects: a minimal model index. J Clin Endocrinol Metab. 2000; 85:4396–4402.20. Steil GM, Hwu CM, Janowski R, Hariri F, Jinagouda S, Darwin C, Tadros S, Rebrin K, Saad MF. Evaluation of insulin sensitivity and beta-cell function indexes obtained from minimal model analysis of a meal tolerance test. Diabetes. 2004; 53:1201–1207.21. Kim M, Oh TJ, Lee JC, Choi K, Kim MY, Kim HC, Cho YM, Kim S. Simulation of oral glucose tolerance tests and the corresponding isoglycemic intravenous glucose infusion studies for calculation of the incretin effect. J Korean Med Sci. 2014; 29:378–385.22. Venkataraman P. Applied optimization with MATLAB programming. 2nd ed. Hoboken: John Wiley & Sons;2009. p. 358.23. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992; 41:368–377.24. Dalla Man C, Yarasheski KE, Caumo A, Robertson H, Toffolo G, Polonsky KS, Cobelli C. Insulin sensitivity by oral glucose minimal models: validation against clamp. Am J Physiol Endocrinol Metab. 2005; 289:E954–E959.25. Breda E, Toffolo G, Polonsky KS, Cobelli C. Insulin release in impaired glucose tolerance: oral minimal model predicts normal sensitivity to glucose but defective response times. Diabetes. 2002; 51:Suppl 1. S227–S233.26. Lee EY, Hwang S, Lee SH, Lee YH, Choi AR, Lee Y, Lee BW, Kang ES, Ahn CW, Cha BS, Lee HC. Postprandial C-peptide to glucose ratio as a predictor of beta-cell function and its usefulness for staged management of type 2 diabetes. J Diabetes Investig. 2014; 5:517–524.27. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999; 22:1462–1470.28. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.29. Chae BN, Lee SK, Hong EG, Chung YS, Lee KW, Kim HM. The role of insulin secretion and insulin resistance in the development of Korean type 2 diabetes mellitus. J Korean Diabetes Assoc. 1998; 22:491–450.30. Oh TJ, Park KS, Cho YM. Correlation of the incretin effect with first- and second-phase insulin secretions in Koreans with various glucose tolerance statuses. Clin Endocrinol (Oxf). 2015; 83:59–66.31. Song J, Oh JY, Sung YA, Pak YK, Park KS, Lee HK. Peripheral blood mitochondrial DNA content is related to insulin sensitivity in offspring of type 2 diabetic patients. Diabetes Care. 2001; 24:865–869.32. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000; 85:2402–2410.33. Otten J, Ahren B, Olsson T. Surrogate measures of insulin sensitivity vs the hyperinsulinaemic-euglycaemic clamp: a meta-analysis. Diabetologia. 2014; 57:1781–1788.34. Reaven GM. What do we learn from measurements of HOMA-IR? Diabetologia. 2013; 56:1867–1868.35. Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Measurement of selective effect of insulin on glucose disposal from labeled glucose oral test minimal model. Am J Physiol Endocrinol Metab. 2005; 289:E909–E914.36. Man CD, Toffolo G, Basu R, Rizza RA, Cobelli C. Use of labeled oral minimal model to measure hepatic insulin sensitivity. Am J Physiol Endocrinol Metab. 2008; 295:E1152–E1159.37. Moller JB, Dalla Man C, Overgaard RV, Ingwersen SH, Tornoe CW, Pedersen M, Tanaka H, Ohsugi M, Ueki K, Lynge J, Vasconcelos NM, Pedersen BK, Kadowaki T, Cobelli C. Ethnic differences in insulin sensitivity, beta-cell function, and hepatic extraction between Japanese and Caucasians: a minimal model analysis. J Clin Endocrinol Metab. 2014; 99:4273–4280.38. Alba M, Ahren B, Inzucchi SE, Guan Y, Mallick M, Xu L, O'Neill EA, Williams-Herman DE, Kaufman KD, Goldstein BJ. Sitagliptin and pioglitazone provide complementary effects on postprandial glucose and pancreatic islet cell function. Diabetes Obes Metab. 2013; 15:1101–1110.39. Dalla Man C, Micheletto F, Sathananthan A, Rizza RA, Vella A, Cobelli C. A model of GLP-1 action on insulin secretion in nondiabetic subjects. Am J Physiol Endocrinol Metab. 2010; 298:E1115–E1121.40. Herrero P, Georgiou P, Oliver N, Reddy M, Johnston D, Toumazou C. A composite model of glucagon-glucose dynamics for in silico testing of bihormonal glucose controllers. J Diabetes Sci Technol. 2013; 7:941–951.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Insulin Secretory Dysfunction in the Pathogenesis of Type 2 Diabetes in Koreans: A Minimal Model Analysis
- Prevalence of Diabetes Mellitus and Associated Diseases in Yeungnam Province Area
- The effect of clofibrate on glucose tolerance in non-insulin-dependent diabetes mellitus patients
- The Insulin Response and Clinical Features According to the Patterns of Glucose Tolerance.
- Plasma Proinsulin Levels among the Control, Impaired Glucose Tolerance and Type 2 Diabetes Mellitus during Oral Glucose Tolerance Test