Association of Single Nucleotide Polymorphisms in Toll-like Receptor Genes With Asthma Risk: a Systematic Review and Meta-analysis
- Affiliations
-
- 1Division of Histology and Immunology, Department of Basic Sciences, Faculty of medicine Tunis, Tunis El Manar University, Tunis, Tunisia. kalttizaoui@gmail.com
- 2Division of Pulmonology, Unit research: 1 2 SP15"Homeostasis and Cell Immune Dysfunction", A. Mami Hospital, Ariana, Tunisia.
- KMID: 2365541
- DOI: http://doi.org/10.4168/aair.2015.7.2.130
Abstract
- PURPOSE
Asthma is a complex disease, with contributions from multiple genes, various genetic backgrounds, and environmental factors. Many human epidemiological studies have demonstrated that single nucleotide polymorphisms (SNPs) in Toll-like receptor (TLR) genes are inconsistently associated with asthma risk. Some have demonstrated differences concerning the study design and effect size, and conflicting results have been reported. A meta-analysis is necessary to determine the magnitude of this association.
METHODS
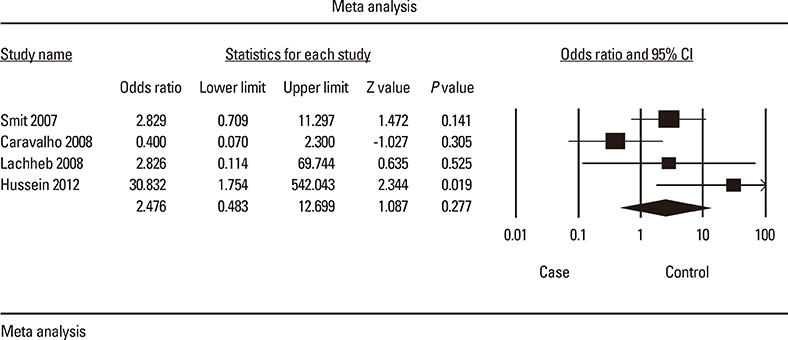
Following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines, a systematic search and meta-analysis of the literature was conducted to estimate the association of SNPs in TLR genes with asthma risk. We screened the medical literature based on the following keyword searches in MEDLINE and EMBASE databases: 'TLR', 'polymorphism', 'asthma', and their combinations.
RESULTS
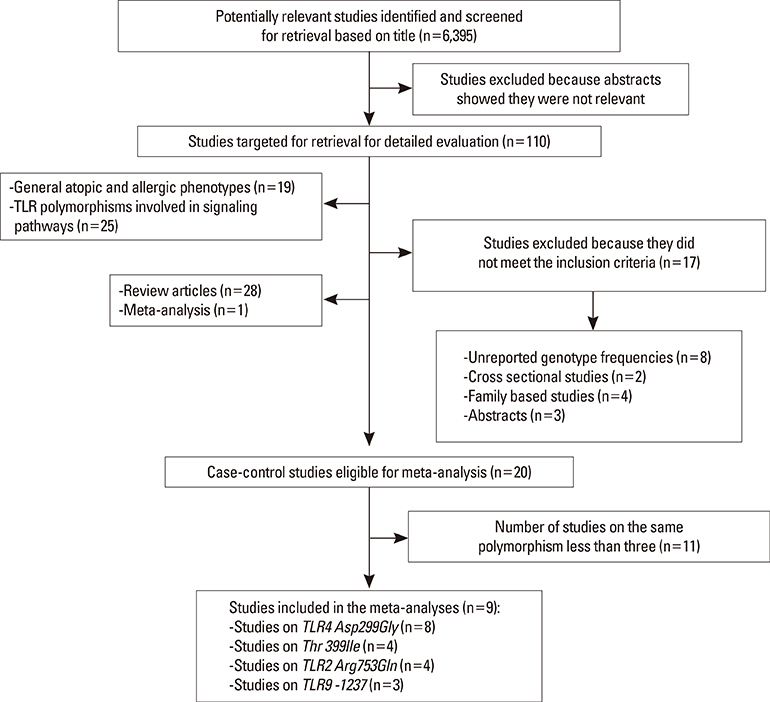
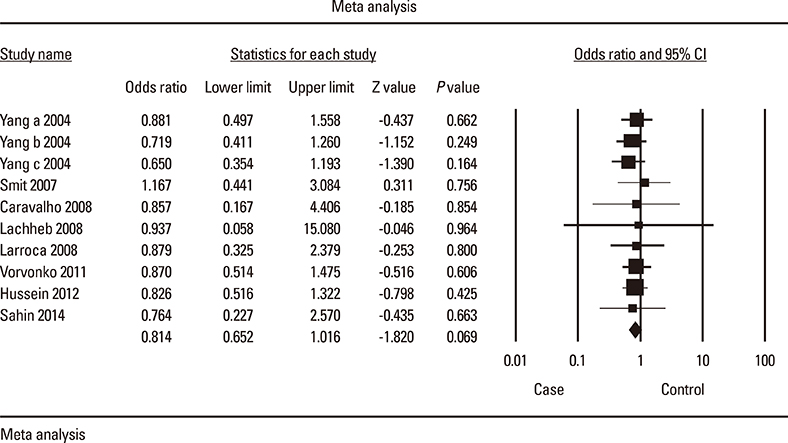
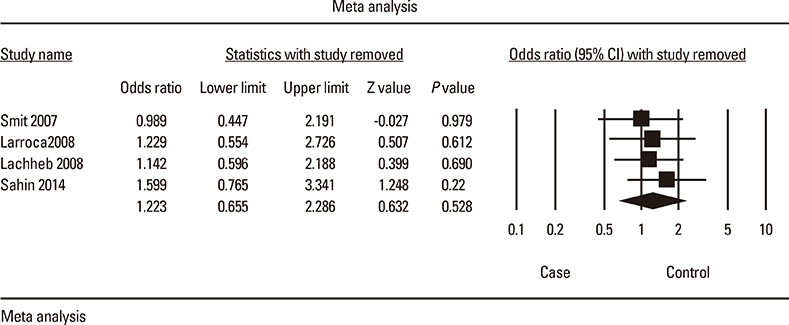
Meta-analysis of eight studies on TLR4 Asp299Gly showed a marginal association of TLR4 with asthma risk (odds ratio [OR]=0.814 [95% confidence interval [CI], 0.652-1.016; P=0.069]) in the recessive model. TLR4 Thr399Ile was not associated with asthma risk under any genetic model. Meta-analysis of four studies on TLR2 Arg753Gln indicated that TLR2 might be significantly associated with asthma in the dominant and codominant models (P=0.029, P=0.030, and P=0.009, respectively). TLR9 -1237 was marginally associated with asthma risk (OR=0.408 [95% CI, 0.163-1.021; P=0.065]) in the codominant model. Analysis using the allele contrast model showed that the major TLR9 -1237 T allele tended to be a significant protective factor with OR=0.689 (95% CI, 0.471-1.007; P=0.055).
CONCLUSIONS
The results showed that TLR4 Asp299Gly, TLR2 Arg753Gln, and TLR9-1237 might contribute significantly to asthma susceptibility. Future genetic association studies would consolidate these findings.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
The Interplay between Host Immunity and Respiratory Viral Infection in Asthma Exacerbation
Ferdaus Mohd Altaf Hossain, Jin Young Choi, Erdenebileg Uyangaa, Seong Ok Park, Seong Kug Eo
Immune Netw. 2019;19(5):. doi: 10.4110/in.2019.19.e31.Investigation of the Possible Role of the Hippo/YAP1 Pathway in Asthma and Allergy
Lili E. Fodor, András Gézsi, ldikó Ungvári, Ágnes F. Semsei, Zsófia Gál, Adrienne Nagy, Gabriella Gálffy, Lilla Tamási, András Kiss, Péter Antal, Csaba Szalai
Allergy Asthma Immunol Res. 2017;9(3):247-256. doi: 10.4168/aair.2017.9.3.247.Allergic Rhinitis in Preschool Children and the Clinical Utility of FeNO
Jisun Yoon, Yean Jung Choi, Eun Lee, Hyun-Ju Cho, Song-I Yang, Young-Ho Kim, Young-Ho Jung, Ju-Hee Seo, Ji-won Kwon, Hyo-Bin Kim, So-Yeon Lee, Bong-Seong Kim, Jung Yeon Shim, Eun-Jin Kim, Joo-Shil Lee, Soo-Jong Hong
Allergy Asthma Immunol Res. 2017;9(4):314-321. doi: 10.4168/aair.2017.9.4.314.
Reference
-
1. Corrigan CJ, Hartnell A, Kay AB. T lymphocyte activation in acute severe asthma. Lancet. 1988; 1:1129–1132.2. Devenny A, Wassall H, Ninan T, Omran M, Khan SD, Russell G. Respiratory symptoms and atopy in children in Aberdeen: questionnaire studies of a defined school population repeated over 35 years. BMJ. 2004; 329:489–490.3. Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol. 2010; 126:453–465.4. Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011; 242:10–30.5. Lee SH, Park JS, Park CS. The search for genetic variants and epigenetics related to asthma. Allergy Asthma Immunol Res. 2011; 3:236–244.6. Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005; 348:1079–1090.7. Phipps S, Lam CE, Foster PS, Matthaei KI. The contribution of toll-like receptors to the pathogenesis of asthma. Immunol Cell Biol. 2007; 85:463–470.8. Kanagaratham C, Camateros P, Flaczyk A, Radzioch D. Polymorphisms in toll-like receptor genes and their roles in allergic asthma and atopy. Recent Pat Inflamm Allergy Drug Discov. 2011; 5:45–56.9. Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005; 17:1–14.10. Liang S, Hosur KB, Lu S, Nawar HF, Weber BR, Tapping RI, et al. Mapping of a microbial protein domain involved in binding and activation of the TLR2/TLR1 heterodimer. J Immunol. 2009; 182:2978–2985.11. Fuchs B, Braun A. Modulation of asthma and allergy by addressing toll-like receptor 2. J Occup Med Toxicol. 2008; 3:Suppl 1. S5.12. Roponen M, Yerkovich ST, Hollams E, Sly PD, Holt PG, Upham JW. Toll-like receptor 7 function is reduced in adolescents with asthma. Eur Respir J. 2010; 35:64–71.13. Stowell NC, Seideman J, Raymond HA, Smalley KA, Lamb RJ, Egenolf DD, et al. Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir Res. 2009; 10:43.14. Lun SW, Wong CK, Ko FW, Hui DS, Lam CW. Expression and functional analysis of toll-like receptors of peripheral blood cells in asthmatic patients: implication for immunopathological mechanism in asthma. J Clin Immunol. 2009; 29:330–342.15. Mizel SB, Honko AN, Moors MA, Smith PS, West AP. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J Immunol. 2003; 170:6217–6223.16. Chun E, Lee SH, Lee SY, Shim EJ, Cho SH, Min KU, et al. Toll-like receptor expression on peripheral blood mononuclear cells in asthmatics; implications for asthma management. J Clin Immunol. 2010; 30:459–464.17. Chuang TH, Ulevitch RJ. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw. 2000; 11:372–378.18. Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004; 303:1529–1531.19. Lazarus R, Raby BA, Lange C, Silverman EK, Kwiatkowski DJ, Vercelli D, et al. Toll-like receptor 10 genetic variation is associated with asthma in two independent samples. Am J Respir Crit Care Med. 2004; 170:594–600.20. Nakashima K, Hirota T, Suzuki Y, Akahoshi M, Shimizu M, Jodo A, et al. Association of the RIP2 gene with childhood atopic asthma. Allergol Int. 2006; 55:77–83.21. Nakashima K, Hirota T, Obara K, Shimizu M, Jodo A, Kameda M, et al. An association study of asthma and related phenotypes with polymorphisms in negative regulator molecules of the TLR signaling pathway. J Hum Genet. 2006; 51:284–291.22. Lee SW, Wang JY, Hsieh YC, Wu YJ, Ting HW, Wu LS. Association of single nucleotide polymorphisms of MD-1 gene with pediatric and adult asthma in the Taiwanese population. J Microbiol Immunol Infect. 2008; 41:445–449.23. Heinzmann A, Brugger M, Bierbaum S, Mailaparambil B, Kopp MV, Strauch K. Joint influences of Acidic-Mammalian-Chitinase with Interleukin-4 and Toll-like receptor-10 with Interleukin-13 in the genetics of asthma. Pediatr Allergy Immunol. 2010; 21:e679–e686.24. Sordillo JE, Sharma S, Poon A, Lasky-Su J, Belanger K, Milton DK, et al. Effects of endotoxin exposure on childhood asthma risk are modified by a genetic polymorphism in ACAA1. BMC Med Genet. 2011; 12:158.25. Smit LA, Bouzigon E, Bousquet J, Le Moual N, Nadif R, Pin I, et al. Mold allergen sensitization in adult asthma according to integrin β3 polymorphisms and Toll-like receptor 2/+596 genotype. J Allergy Clin Immunol. 2011; 128:185–191.e7.26. Bjørnvold M, Munthe-Kaas MC, Egeland T, Joner G, Dahl-Jørgensen K, Njølstad PR, et al. A TLR2 polymorphism is associated with type 1 diabetes and allergic asthma. Genes Immun. 2009; 10:181–187.27. Qian FH, Zhang Q, Zhou LF, Jin GF, Bai JL, Yin KS. Polymorphisms in the toll-like receptor 2 subfamily and risk of asthma: a case-control analysis in a Chinese population. J Investig Allergol Clin Immunol. 2010; 20:340–346.28. Hussein YM, Awad HA, Shalaby SM, Ali AS, Alzahrani SS. Toll-like receptor 2 and Toll-like receptor 4 polymorphisms and susceptibility to asthma and allergic rhinitis: a case-control analysis. Cell Immunol. 2012; 274:34–38.29. Zhang Q, Qian FH, Zhou LF, Wei GZ, Jin GF, Bai JL, et al. Polymorphisms in toll-like receptor 4 gene are associated with asthma severity but not susceptibility in a Chinese Han population. J Investig Allergol Clin Immunol. 2011; 21:370–377.30. Tantisira K, Klimecki WT, Lazarus R, Palmer LJ, Raby BA, Kwiatkowski DJ, et al. Toll-like receptor 6 gene (TLR6): single-nucleotide polymorphism frequencies and preliminary association with the diagnosis of asthma. Genes Immun. 2004; 5:343–346.31. Zhang Q, Qian F, Zhou L, Wei G, Wang Y, Hu Z, et al. Polymorphisms of TLR7 and TLR8 associated with risk of asthma and asthma-related phenotypes in a southeastern Chinese Han population. J Nanjing Med Univ. 2009; 23:25–32.32. Lachheb J, Dhifallah IB, Chelbi H, Hamzaoui K, Hamzaoui A. Toll-like receptors and CD14 genes polymorphisms and susceptibility to asthma in Tunisian children. Tissue Antigens. 2008; 71:417–425.33. Lazarus R, Klimecki WT, Raby BA, Vercelli D, Palmer LJ, Kwiatkowski DJ, et al. Single-nucleotide polymorphisms in the Toll-like receptor 9 gene (TLR9): frequencies, pairwise linkage disequilibrium, and haplotypes in three U.S. ethnic groups and exploratory case-control disease association studies. Genomics. 2003; 81:85–91.34. Lazarus R, Vercelli D, Palmer LJ, Klimecki WJ, Silverman EK, Richter B, et al. Single nucleotide polymorphisms in innate immunity genes: abundant variation and potential role in complex human disease. Immunol Rev. 2002; 190:9–25.35. Leung TF, Tang NL, Wong GW, Fok TF. CD14 and toll-like receptors: potential contribution of genetic factors and mechanisms to inflammation and allergy. Curr Drug Targets Inflamm Allergy. 2005; 4:169–175.36. Yang IA, Fong KM, Holgate ST, Holloway JW. The role of Toll-like receptors and related receptors of the innate immune system in asthma. Curr Opin Allergy Clin Immunol. 2006; 6:23–28.37. Daley D, Park JE, He JQ, Yan J, Akhabir L, Stefanowicz D, et al. Associations and interactions of genetic polymorphisms in innate immunity genes with early viral infections and susceptibility to asthma and asthma-related phenotypes. J Allergy Clin Immunol. 2012; 130:1284–1293.38. Miedema KG, Tissing WJ, Te Poele EM, Kamps WA, Alizadeh BZ, Kerkhof M, et al. Polymorphisms in the TLR6 gene associated with the inverse association between childhood acute lymphoblastic leukemia and atopic disease. Leukemia. 2012; 26:1203–1210.39. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010; 8:336–341.40. Light RJ, Singer JD, Willett JB. The visual presentation and interpretation of meta-analyses. In : Cooper H, Hedges LV, editors. The handbook of research synthesis. New York (NY): Russell Sage Foundation;1994. p. 439–454.41. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954; 10:101–129.42. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.43. Saçkesen C, Karaaslan C, Keskin O, Tokol N, Tahan F, Civelek E, et al. The effect of polymorphisms at the CD14 promoter and the TLR4 gene on asthma phenotypes in Turkish children with asthma. Allergy. 2005; 60:1485–1492.44. Puthothu B, Heinzmann A. Is toll-like receptor 6 or toll-like receptor 10 involved in asthma genetics--or both? Allergy. 2006; 61:649–650.45. Kormann MS, Depner M, Hartl D, Klopp N, Illig T, Adamski J, et al. Toll-like receptor heterodimer variants protect from childhood asthma. J Allergy Clin Immunol. 2008; 122:86–92. 92.e1–92.e8.46. Smit LA, Siroux V, Bouzigon E, Oryszczyn MP, Lathrop M, Demenais F, et al. CD14 and toll-like receptor gene polymorphisms, country living, and asthma in adults. Am J Respir Crit Care Med. 2009; 179:363–368.47. Kerkhof M, Postma DS, Brunekreef B, Reijmerink NE, Wijga AH, de Jongste JC, et al. Toll-like receptor 2 and 4 genes influence susceptibility to adverse effects of traffic-related air pollution on childhood asthma. Thorax. 2010; 65:690–697.48. Fagerås Böttcher M, Hmani-Aifa M, Lindström A, Jenmalm MC, Mai XM, Nilsson L, et al. A TLR4 polymorphism is associated with asthma and reduced lipopolysaccharide-induced interleukin-12(p70) responses in Swedish children. J Allergy Clin Immunol. 2004; 114:561–567.49. Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrländer C, et al. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004; 113:482–488.50. Raby BA, Klimecki WT, Laprise C, Renaud Y, Faith J, Lemire M, et al. Polymorphisms in toll-like receptor 4 are not associated with asthma or atopy-related phenotypes. Am J Respir Crit Care Med. 2002; 166:1449–1456.51. Werner M, Topp R, Wimmer K, Richter K, Bischof W, Wjst M, et al. TLR4 gene variants modify endotoxin effects on asthma. J Allergy Clin Immunol. 2003; 112:323–330.52. Møller-Larsen S, Nyegaard M, Haagerup A, Vestbo J, Kruse TA, Børglum AD. Association analysis identifies TLR7 and TLR8 as novel risk genes in asthma and related disorders. Thorax. 2008; 63:1064–1069.53. Lange NE, Zhou X, Lasky-Su J, Himes BE, Lazarus R, Soto-Quirós M, et al. Comprehensive genetic assessment of a functional TLR9 promoter polymorphism: no replicable association with asthma or asthma-related phenotypes. BMC Med Genet. 2011; 12:26.54. Adjers K, Karjalainen J, Pessi T, Eklund C, Hurme M. Epistatic effect of TLR4 and IL4 genes on the risk of asthma in females. Int Arch Allergy Immunol. 2005; 138:251–256.55. He J, Bosse Y, Laprise C, Paré P, Sandford A, Kozyrskyj A, et al. Novel associations of genetic polymorphisms in the interleukin-1 receptor/Toll-like receptor signaling pathways with atopy and atopic asthma. J Allergy Clin Immunol. 2009; 123:S167.56. Koponen P, Vuononvirta J, Nuolivirta K, Helminen M, He Q, Korppi M. The association of genetic variants in toll-like receptor 2 subfamily with allergy and asthma after hospitalization for bronchiolitis in infancy. Pediatr Infect Dis J. 2014; 33:463–466.57. Noguchi E, Nishimura F, Fukai H, Kim J, Ichikawa K, Shibasaki M, et al. An association study of asthma and total serum immunoglobin E levels for Toll-like receptor polymorphisms in a Japanese population. Clin Exp Allergy. 2004; 34:177–183.58. Yang IA, Barton SJ, Rorke S, Cakebread JA, Keith TP, Clough JB, et al. Toll-like receptor 4 polymorphism and severity of atopy in asthmatics. Genes Immun. 2004; 5:41–45.59. Hoffjan S, Stemmler S, Parwez Q, Petrasch-Parwez E, Arinir U, Rohde G, et al. Evaluation of the toll-like receptor 6 Ser249Pro polymorphism in patients with asthma, atopic dermatitis and chronic obstructive pulmonary disease. BMC Med Genet. 2005; 6:34.60. Smit LA, Bongers SI, Ruven HJ, Rijkers GT, Wouters IM, Heederik D, et al. Atopy and new-onset asthma in young Danish farmers and CD14, TLR2, and TLR4 genetic polymorphisms: a nested case-control study. Clin Exp Allergy. 2007; 37:1602–1608.61. Carvalho A, Pasqualotto AC, Pitzurra L, Romani L, Denning DW, Rodrigues F. Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis. 2008; 197:618–621.62. Larocca N, DeSanctis J, Toro F, Moreno D. F.23. Polymorphisms of Toll-like receptor 2 and 4 genes in asthma and COPD. Clin Immunol. 2006; 119:Suppl. S58–S59.63. Hsieh YY, Wan L, Chang CC, Tsai CH, Tsai FJ. STAT2*C related genotypes and allele but not TLR4 and CD40 gene polymorphisms are associated with higher susceptibility for asthma. Int J Biol Sci. 2009; 5:74–81.64. Qian XB, Wu Y, Cao SY, Cai XH, Yu CY, Xuan MY, et al. Association of single nucleotide polymorphisms in the promoter region of the TLR9 gene with childhood atopic asthma. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2011; 28:185–189.65. Palikhe NS, Kim SH, Kim JH, Losol P, Ye YM, Park HS. Role of toll-like receptor 3 variants in aspirin-exacerbated respiratory disease. Allergy Asthma Immunol Res. 2011; 3:123–127.66. Voron'ko OE, Dmitrieva-Zdorova EV, Latysheva EA, Aksenova MG, Storozhakov GI, Bodoev NV, et al. CARD15 and TLR4 genes polymorphisms in atopic bronchial asthma. Mol Biol (Mosk). 2011; 45:831–839.67. Sahin F, Yıldız P, Kuskucu A, Kuskucu MA, Karaca N, Midilli K. The effect of CD14 and TLR4 gene polimorphisms on asthma phenotypes in adult Turkish asthma patients: a genetic study. BMC Pulm Med. 2014; 14:20.68. Pacheco-Martínez MM, Saucedo-Ramírez O, Del Rio Navarro B, Del Rio-Chivardi J, Cruz M, Pérez-Figueroa GE, et al. Toll-like receptor 4 expression in obese asthmatic children with allergic inflammation. Bol Med Hosp Infant Mex. 2011; 68:257–261.69. Liang XH, Cheung W, Heng CK, Wang DY. Absence of the toll-like receptor 4 gene polymorphisms Asp299Gly and Thr399Ile in Singaporean Chinese. Ther Clin Risk Manag. 2005; 1:243–246.70. Douville RN, Lissitsyn Y, Hirschfeld AF, Becker AB, Kozyrskyj AL, Liem J, et al. TLR4 Asp299Gly and Thr399Ile polymorphisms: no impact on human immune responsiveness to LPS or respiratory syncytial virus. PLoS One. 2010; 5:e12087.71. Chen S. Association between the TLR4 +896A>G (Asp299Gly) polymorphism and asthma: a systematic review and meta-analysis. J Asthma. 2012; 49:999–1003.72. Klaassen EM, Thönissen BE, van Eys G, Dompeling E, Jöbsis Q. A systematic review of CD14 and toll-like receptors in relation to asthma in Caucasian children. Allergy Asthma Clin Immunol. 2013; 9:10.73. Marth G, Yeh R, Minton M, Donaldson R, Li Q, Duan S, et al. Single-nucleotide polymorphisms in the public domain: how useful are they? Nat Genet. 2001; 27:371–372.74. Trajkov D, Mirkovska-Stojkovikj J, Arsov T, Petlichkovski A, Strezova A, Efinska-Mladenovska O, et al. Association of cytokine gene polymorphisms with bronchial asthma in Macedonians. Iran J Allergy Asthma Immunol. 2008; 7:143–156.75. Saadat M, Ansari-Lari M. Genetic polymorphism of glutathione S-transferase T1, M1 and asthma, a meta-analysis of the literature. Pak J Biol Sci. 2007; 10:4183–4189.76. Cui L, Jia J, Ma CF, Li SY, Wang YP, Guo XM, et al. IL-13 polymorphisms contribute to the risk of asthma: a meta-analysis. Clin Biochem. 2012; 45:285–288.77. Piacentini S, Polimanti R, Simonelli I, Donno S, Pasqualetti P, Manfellotto D, et al. Glutathione S-transferase polymorphisms, asthma susceptibility and confounding variables: a meta-analysis. Mol Biol Rep. 2013; 40:3299–3313.78. Lee YH, Choi SJ, Ji JD, Song GG. The CTLA-4 +49 A/G and -318 C/T polymorphisms and susceptibility to asthma: a meta-analysis. Mol Biol Rep. 2012; 39:8525–8532.79. von Mutius E. Gene-environment interactions in asthma. J Allergy Clin Immunol. 2009; 123:3–11.80. Bezemer GF, Sagar S, van Bergenhenegouwen J, Georgiou NA, Garssen J, Kraneveld AD, et al. Dual role of toll-like receptors in asthma and chronic obstructive pulmonary disease. Pharmacol Rev. 2012; 64:337–358.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Single Nucleotide Polymorphisms of Toll-Like Receptor 7 and Toll-Like Receptor 9 in Hepatitis C Virus Infection Patients from Central China
- Polymorphisms of Inflammatory Cytokine Genesand Risk for Intracranial Aneurysm:A Systematic Review and Meta-Analysis
- Association of Toll-Like Receptor 5 Gene Polymorphism with Susceptibility to Ossification of the Posterior Longitudinal Ligament of the Spine in Korean Population
- Eosinophilic Bronchitis, Eosinophilia Associated Genetic Variants, and Notch Signaling in Asthma
- Association between Polymorphisms in Toll-like Receptor 9 Gene and Outcomes after Ischemic Stroke