Korean J Physiol Pharmacol.
2016 Nov;20(6):641-647. 10.4196/kjpp.2016.20.6.641.
Hypoxic pulmonary vasoconstriction and vascular contractility in monocrotaline-induced pulmonary arterial hypertensive rats
- Affiliations
-
- 1Department of Physiology, Department of Biomedical Sciences and Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul 03080, Korea.
- 2Chung-Ang University Red Cross College of Nursing, Seoul 06974, Korea. hyoo@cau.ac.kr
- KMID: 2364268
- DOI: http://doi.org/10.4196/kjpp.2016.20.6.641
Abstract
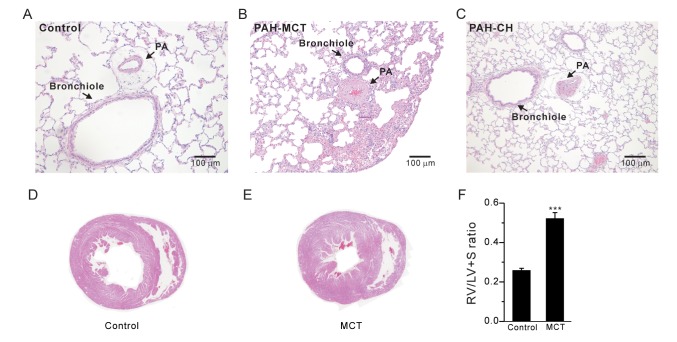
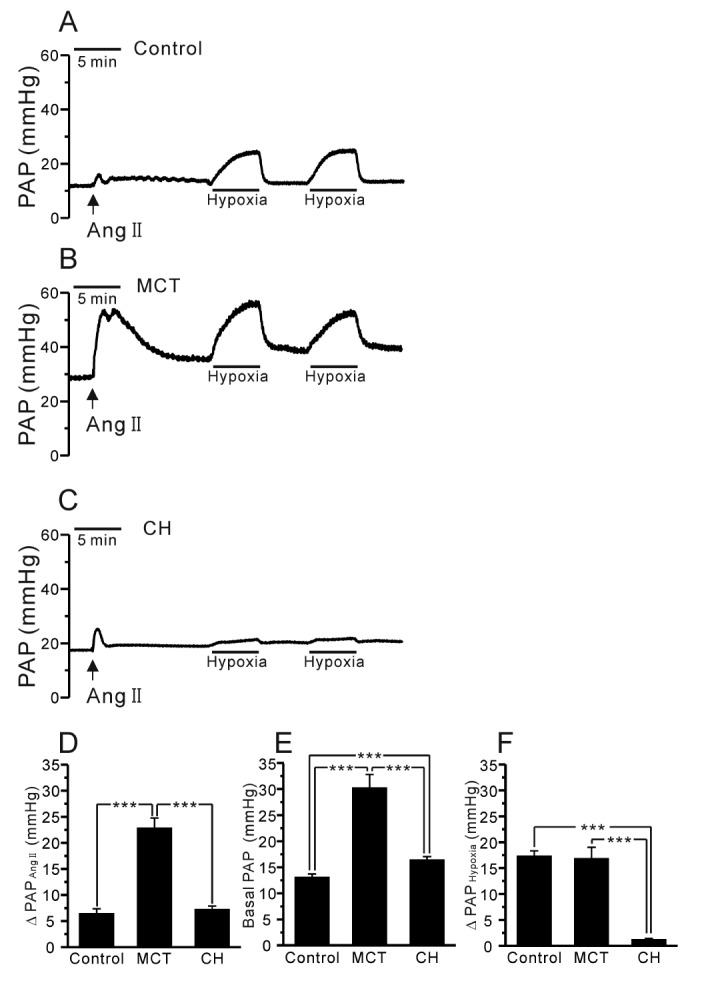
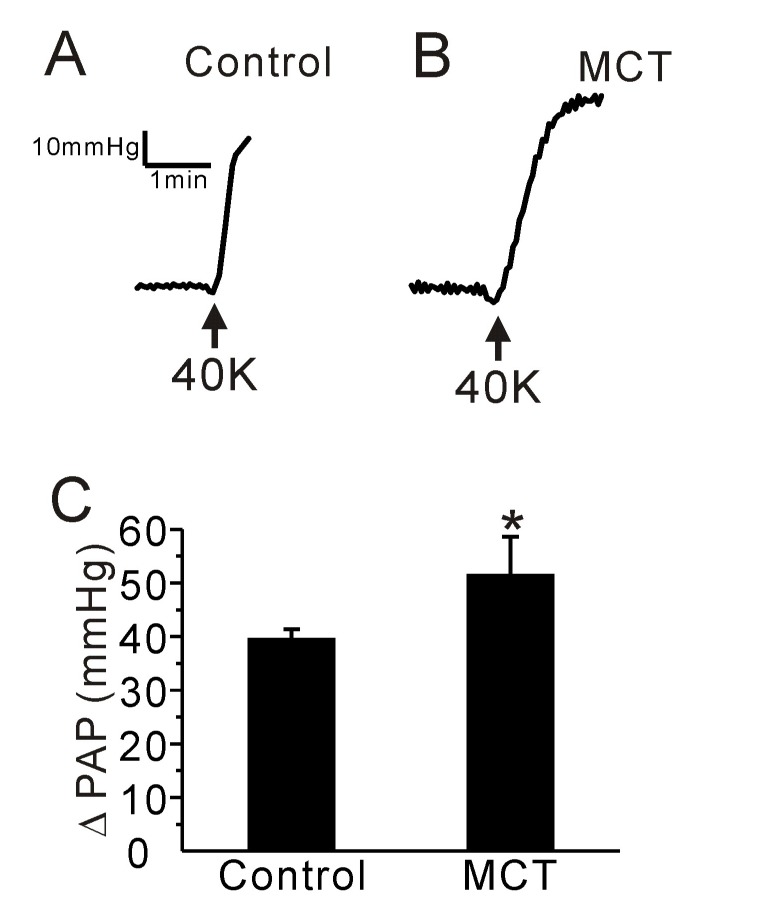
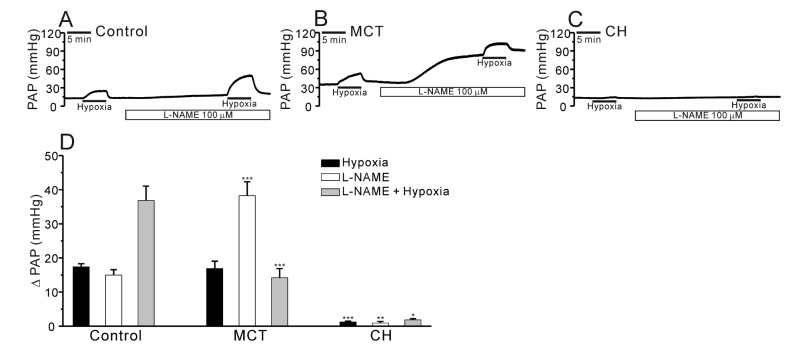
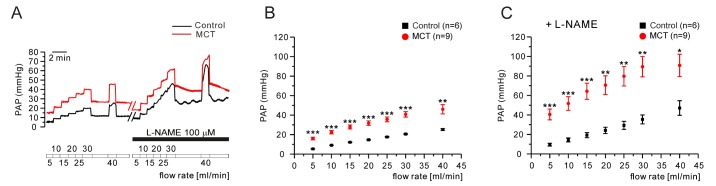
- Pulmonary arterial hypertension (PAH) is a progressive disease characterized by vascular remodeling of pulmonary arteries (PAs) and increased vascular resistance in the lung. Monocrotaline (MCT), a toxic alkaloid, is widely used for developing rat models of PAH caused by injury to pulmonary endothelial cells; however, characteristics of vascular functions in MCT-induced PAH vary and are not fully understood. Here, we investigated hypoxic pulmonary vasoconstriction (HPV) responses and effects of various vasoconstrictors with isolated/perfused lungs of MCT-induced PAH (PAH-MCT) rats. Using hematoxylin and eosin staining, we confirmed vascular remodeling (i.e., medial thickening of PA) and right ventricle hypertrophy in PAH-MCT rats. The basal pulmonary arterial pressure (PAP) and PAP increase by a raised flow rate (40 mL/min) were higher in the PAH-MCT than in the control rats. In addition, both high K⺠(40 mM KCl)- and angiotensin II-induced PAP increases were higher in the PAH-MCT than in the control rats. Surprisingly, application of a nitric oxide synthase inhibitor, L-N(G)-Nitroarginine methyl ester (L-NAME), induced a marked PAP increase in the PAH-MCT rats, suggesting that endothelial functions were recovered in the three-week PAH-MCT rats. In addition, the medial thickening of the PA was similar to that in chronic hypoxia-induced PAH (PAH-CH) rats. However, the HPV response (i.e., PAP increased by acute hypoxia) was not affected in the MCT rats, whereas HPV disappeared in the PAH-CH rats. These results showed that vascular contractility and HPV remain robust in the MCT-induced PAH rat model with vascular remodeling.
Keyword
MeSH Terms
-
Angiotensins
Animals
Anoxia
Arterial Pressure
Endothelial Cells
Eosine Yellowish-(YS)
Heart Ventricles
Hematoxylin
Hypertension
Hypertrophy
Lung
Models, Animal
Monocrotaline
Nitric Oxide Synthase
Pulmonary Artery
Rats*
Vascular Remodeling
Vascular Resistance
Vasoconstriction*
Vasoconstrictor Agents
Angiotensins
Eosine Yellowish-(YS)
Hematoxylin
Monocrotaline
Nitric Oxide Synthase
Vasoconstrictor Agents
Figure
Cited by 2 articles
-
Decreased inward rectifier and voltage-gated K+ currents of the right septal coronary artery smooth muscle cells in pulmonary arterial hypertensive rats
Sung Eun Kim, Ming Zhe Yin, Hae Jin Kim, Rany Vorn, Hae Young Yoo, Sung Joon Kim
Korean J Physiol Pharmacol. 2020;24(1):111-119. doi: 10.4196/kjpp.2020.24.1.111.Inhibitory effects of 2,6-di-
tert -butyl-4-hydroxymethylphenol on asthmatic responses to ovalbumin challenge in conscious guinea pigs
Seul-Yong Jeong, Ji-Yun Lee
Korean J Physiol Pharmacol. 2018;22(1):81-89. doi: 10.4196/kjpp.2018.22.1.81.
Reference
-
1. Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, Gaine S. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004; 43(12 Suppl S):40S–47S. PMID: 15194177.
Article2. Leopold JA, Maron BA. Molecular mechanisms of pulmonary vascular remodeling in pulmonary arterial hypertension. Int J Mol Sci. 2016; 17:pii: E761.
Article3. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009; 297:L1013–L1032. PMID: 19748998.
Article4. Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res. 2009; 10:95. PMID: 19825167.
Article5. Sumpio BE, Riley JT, Dardik A. Cells in focus: endothelial cell. Int J Biochem Cell Biol. 2002; 34:1508–1512. PMID: 12379270.
Article6. Zhang S, Dong H, Rubin LJ, Yuan JX. Upregulation of Na+/Ca2+ exchanger contributes to the enhanced Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2007; 292:C2297–C2305. PMID: 17192285.7. de Jesus Perez VA. Molecular pathogenesis and current pathology of pulmonary hypertension. Heart Fail Rev. 2016; 21:239–257. PMID: 26694808.
Article8. Colvin KL, Yeager ME. Animal Models of Pulmonary Hypertension: Matching Disease Mechanisms to Etiology of the Human Disease. J Pulm Respir Med. 2014; 4:pii: 198.
Article9. Zhao LR, Nam SC. Multiphoton microscope imaging: the behavior of neural progenitor cells in the rostral migratory stream. Neurosci Lett. 2007; 425:83–88. PMID: 17723276.
Article10. Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004; 287:L656–L664. PMID: 14977625.
Article11. Morimatsu Y, Sakashita N, Komohara Y, Ohnishi K, Masuda H, Dahan D, Takeya M, Guibert C, Marthan R. Development and characterization of an animal model of severe pulmonary arterial hypertension. J Vasc Res. 2012; 49:33–42. PMID: 21985792.
Article12. Wilkins MR, Wharton J, Zhao L. What animal models tell us about treatments for pulmonary hypertension. In : Antel J, Hesselink MB, Schermuly RT, editors. Pulmonary arterial hypertension. Amsterdam: IOS Press;2010. p. 57–69.13. Frasch HF, Marshall C, Marshall BE. Endothelin-1 is elevated in monocrotaline pulmonary hypertension. Am J Physiol. 1999; 276:L304–L310. PMID: 9950893.14. Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L363–L369. PMID: 21964406.
Article15. Archer SL, Wu XC, Thébaud B, Nsair A, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res. 2004; 95:308–318. PMID: 15217912.16. Osipenko ON, Tate RJ, Gurney AM. Potential role for kv3.1b channels as oxygen sensors. Circ Res. 2000; 86:534–540. PMID: 10720415.
Article17. Yoo HY, Park SJ, Seo EY, Park KS, Han JA, Kim KS, Shin DH, Earm YE, Zhang YH, Kim SJ. Role of thromboxane A2-activated nonselective cation channels in hypoxic pulmonary vasoconstriction of rat. Am J Physiol Cell Physiol. 2012; 302:C307–C317. PMID: 21998141.18. Weissmann N, Nollen M, Gerigk B, Ardeschir Ghofrani H, Schermuly RT, Gunther A, Quanz K, Fink L, Hänze J, Rose F, Seeger W, Grimminger F. Downregulation of hypoxic vasoconstriction by chronic hypoxia in rabbits: effects of nitric oxide. Am J Physiol Heart Circ Physiol. 2003; 284:H931–H938. PMID: 12433654.19. Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med. 2013; 187:424–432. PMID: 23328522.
Article20. Mathew R, Gloster ES, Sundararajan T, Thompson CI, Zeballos GA, Gewitz MH. Role of inhibition of nitric oxide production in monocrotaline-induced pulmonary hypertension. J Appl Physiol (1985). 1997; 82:1493–1498. PMID: 9134898.
Article21. Liu CP, Dai ZK, Huang CH, Yeh JL, Wu BN, Wu JR, Chen IJ. Endothelial nitric oxide synthase-enhancing G-protein coupled receptor antagonist inhibits pulmonary artery hypertension by endothelin-1-dependent and endothelin-1-independent pathways in a monocrotaline model. Kaohsiung J Med Sci. 2014; 30:267–278. PMID: 24835346.
Article22. Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003; 107:2037–2044. PMID: 12695303.23. Reeve HL, Michelakis E, Nelson DP, Weir EK, Archer SL. Alterations in a redox oxygen sensing mechanism in chronic hypoxia. J Appl Physiol (1985). 2001; 90:2249–2256. PMID: 11356790.
Article24. Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006; 99:675–691. PMID: 17008597.25. Yoo HY, Kim SJ. Disappearance of hypoxic pulmonary vasoconstriction and o2-sensitive nonselective cationic current in arterial myocytes of rats under ambient hypoxia. Korean J Physiol Pharmacol. 2013; 17:463–468. PMID: 24227949.26. Schwenke DO, Gray EA, Pearson JT, Sonobe T, Ishibashi-Ueda H, Campillo I, Kangawa K, Umetani K, Shirai M. Exogenous ghrelin improves blood flow distribution in pulmonary hypertension-assessed using synchrotron radiation microangiography. Pflugers Arch. 2011; 462:397–406. PMID: 21744075.
Article27. van Suylen RJ, Smits JF, Daemen MJ. Pulmonary artery remodeling differs in hypoxia- and monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med. 1998; 157:1423–1428. PMID: 9603118.
Article28. Kay JM, Suyama KL, Keane PM. Failure to show decrease in small pulmonary blood vessels in rats with experimental pulmonary hypertension. Thorax. 1982; 37:927–930. PMID: 6820578.
Article29. Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009; 135:794–804. PMID: 19265089.30. Chuang IC, Yang RC, Chou SH, Huang LR, Tsai TN, Dong HP, Huang MS. Effect of carbon dioxide inhalation on pulmonary hypertension induced by increased blood flow and hypoxia. Kaohsiung J Med Sci. 2011; 27:336–343. PMID: 21802645.
Article31. Galié N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc Res. 2004; 61:227–237. PMID: 14736539.32. Schwenke DO, Pearson JT, Sonobe T, Ishibashi-Ueda H, Shimouchi A, Kangawa K, Umetani K, Shirai M. Role of Rho-kinase signaling and endothelial dysfunction in modulating blood flow distribution in pulmonary hypertension. J Appl Physiol (1985). 2011; 110:901–908. PMID: 21212241.
Article33. de Man FS, Tu L, Handoko ML, Rain S, Ruiter G, François C, Schalij I, Dorfmüller P, Simonneau G, Fadel E, Perros F, Boonstra A, Postmus PE, van der, Vonk-Noordegraaf A, Humbert M, Eddahibi S, Guignabert C. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012; 186:780–789. PMID: 22859525.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Intra - Pulmonary Arterial Diltiazem on Hypoxic Pulmonary Vasoconstriction in Dogs
- Pulmonary Hypertension Associated with Acute Hypoxic Pulmonary Vasoconstriction in a Patient with Acute Myeloid Leukemia
- ED50 of Verapamil Inhibiting Hypoxic Pulmonary Vasoconstriction in Rats
- A Morphological Study of the Pulmonary Endothelium and Neuroendocrine Cells in Monocrotaline-Induced Pulmonary Arterial Hypertension
- Pathophysiology of Persistent Pulmonary Hypertension of the Newborn