Allergy Asthma Respir Dis.
2016 Mar;4(2):91-99. 10.4168/aard.2016.4.2.91.
Clinicoepidemiological research designs in childhood allergic diseases
- Affiliations
-
- 1Department of Pediatrics, Soonchunhyang University Hospital, Soonchunhyang University College of Medicine, Seoul, Korea. Ilove902@hanmail.net
- KMID: 2361280
- DOI: http://doi.org/10.4168/aard.2016.4.2.91
Abstract
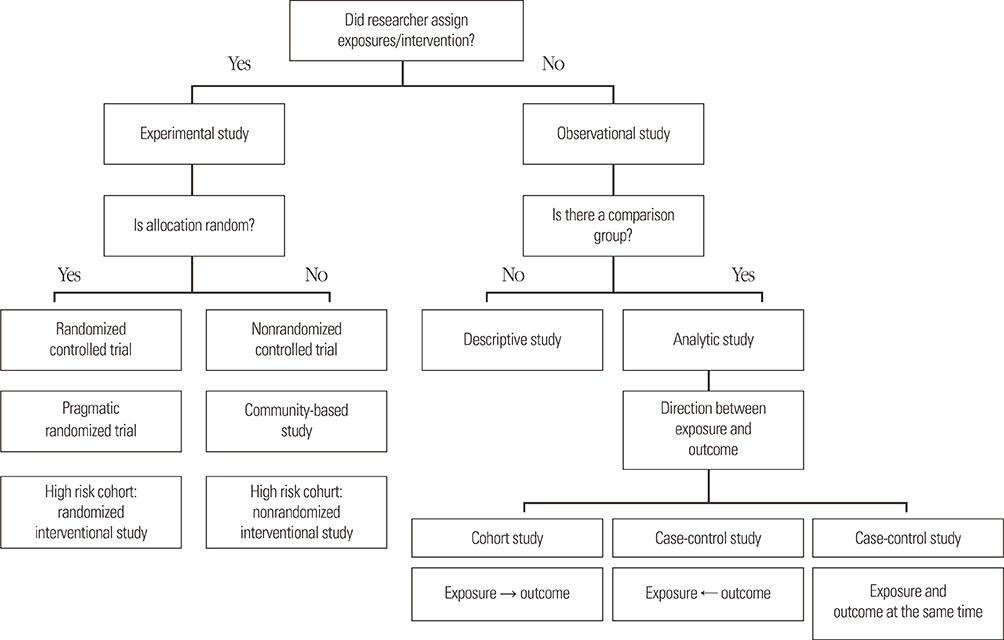
- Clinical epidemiology is defined as a method for investigating the distribution and determinants of diseases and for applying this knowledge in their prevention, and simply means application of epidemiological methods for medical research. In evidence-based medicine, randomized controlled trials (RCT) are the gold standard for assessing efficacy and safety of the intervention, while it is commonly impractical because of many limitations, such as ethical/legal problems and weak external-validity. High internal-validity of RCT permits to assess the direct efficacy of intervention without interference with bias and confounder; however, it has less generalizability or applicability to the real-life practice. Evidence-based practical guidelines are developed for patient management and decision making in real-life practice; paradoxically, the evidence of the guidelines does not come from real life, but from strict trial life. To overcome these limitations, pragmatic clinical trials for assessing the effectiveness of intervention in real-life practice or high-quality observational studies would be the best alternatives or could add more strong evidence. This article provides an overview of clinicoepidemiological research designs in the field of childhood allergic diseases and their strength/weakness.
Keyword
MeSH Terms
Figure
Reference
-
1. Rueter K, Haynes A, Prescott SL. Developing primary intervention strategies to prevent allergic disease. Curr Allergy Asthma Rep. 2015; 15:40.
Article2. Caminati M, Durie-Filipovie I, Arasi S, Peroni DG, Zivkovie Z, Senna G. Respiratory allergies in childhood: Recent advances and future challenges. Pediatr Allergy Immunol. 2015; 26:702–710.
Article3. Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet. 2015; 386:1075–1085.
Article4. Yang HJ, Lee SY, Suh DI, Shin YH, Kim BJ, Seo JH, et al. The Cohort for Childhood Origin of Asthma and allergic diseases (COCOA) study: design, rationale and methods. BMC Pulm Med. 2014; 14:109.
Article5. Nath R, Ahuja RC, Kumar S. Clinical epidemiology: what, why and how? Indian J Ophthalmol. 1995; 43:83–87.6. Iwanaga T, Tohda Y. Importance of clinical epidemiology research in studies on respiratory diseases. Respir Investig. 2013; 51:217–223.
Article7. Roche N, Reddel HK, Agusti A, Bateman ED, Krishnan JA, Martin RJ, et al. Integrating real-life studies in the global therapeutic research framework. Lancet Respir Med. 2013; 1:e29–e30.
Article8. Price D, Smith P, Hellings P, Papadopoulos N, Fokkens W, Muraro A, et al. Current controversies and challenges in allergic rhinitis management. Expert Rev Clin Immunol. 2015; 11:1205–1217.
Article9. Price D, Brusselle G, Roche N, Freeman D, Chisholm A. Real-world research and its importance in respiratory medicine. Breathe (Sheff). 2015; 11:26–38.
Article10. Rivas-Ruiz F, Exposito-Ruiz M, Dominguez-Almendros S. Research designs in clinical epidemiology. Allergol Immunopathol (Madr). 2012; 40:117–124.
Article11. Röhrig B, du Prel JB, Wachtlin D, Blettner M. Types of study in medical research: part 3 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2009; 106:262–268.12. Beasley R, Clayton T, Crane J, von Mutius E, Lai CK, Montefort S, et al. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6-7 years: analysis from Phase Three of the ISAAC programme. Lancet. 2008; 372:1039–1048.
Article13. Singh M. Paracetamol as a risk factor for allergic disorders. Lancet. 2009; 373:119.
Article14. Lawrence J, Moore E, Port L, Danchin M, Connell T. Paracetamol as a risk factor for allergic disorders. Lancet. 2009; 373:119.
Article15. Sordillo JE, Scirica CV, Rifas-Shiman SL, Gillman MW, Bunyavanich S, Camargo CA Jr, et al. Prenatal and infant exposure to acetaminophen and ibuprofen and the risk for wheeze and asthma in children. J Allergy Clin Immunol. 2015; 135:441–448.
Article16. Cheelo M, Lodge CJ, Dharmage SC, Simpson JA, Matheson M, Heinrich J, et al. Paracetamol exposure in pregnancy and early childhood and development of childhood asthma: a systematic review and meta-analysis. Arch Dis Child. 2015; 100:81–89.
Article17. Yang HJ, Qin R, Katusic S, Juhn YJ. Population-based study on association between birth weight and risk of asthma: a propensity score approach. Ann Allergy Asthma Immunol. 2013; 110:18–23.
Article18. Cai T, Zheng Y. Evaluating prognostic accuracy of biomarkers in nested case-control studies. Biostatistics. 2012; 13:89–100.
Article19. Ortqvist AK, Lundholm C, Carlström E, Lichtenstein P, Cnattingius S, Almqvist C. Familial factors do not confound the association between birth weight and childhood asthma. Pediatrics. 2009; 124:e737–e743.20. DiPietro NA. Methods in epidemiology: observational study designs. Pharmacotherapy. 2010; 30:973–984.
Article21. Magnus MC, DeRoo LA, Haberg SE, Magnus P, Nafstad P, Nystad W, et al. Prospective study of maternal alcohol intake during pregnancy or lactation and risk of childhood asthma: the Norwegian Mother and Child Cohort Study. Alcohol Clin Exp Res. 2014; 38:1002–1011.
Article22. Covar RA, Fuhlbrigge AL, Williams P, Kelly HW. the Childhood Asthma Management Program Research Group. The Childhood Asthma Management Program (CAMP): Contributions to the Understanding of Therapy and the Natural History of Childhood Asthma. Curr Respir Care Rep. 2012; 1:243–250.
Article23. Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012; 367:904–912.
Article24. Newcomb DC, Cephus JY, Boswell MG, Fahrenholz JM, Langley EW, Feldman AS, et al. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J Allergy Clin Immunol. 2015; 136:1025–1034.e11.
Article25. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010; 181:315–323.
Article26. Bousquet J, Gern JE, Martinez FD, Anto JM, Johnson CC, Holt PG, et al. Birth cohorts in asthma and allergic diseases: report of a NIAID/NHLBI/MeDALL joint workshop. J Allergy Clin Immunol. 2014; 133:1535–1546.
Article27. Kim KW, Ahn K, Yang HJ, Lee S, Park JD, Kim WK, et al. Humidifier disinfectant-associated children's interstitial lung disease. Am J Respir Crit Care Med. 2014; 189:48–56.28. Yang HJ, Kim HJ, Yu J, Lee E, Jung YH, Kim HY, et al. Inhalation toxicity of humidifier disinfectants as a risk factor of children's interstitial lung disease in Korea: a case-control study. PLoS One. 2013; 8:e64430.
Article29. Been JV, Nurmatov UB, Cox B, Nawrot TS, van Schayck CP, Sheikh A. Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis. Lancet. 2014; 383:1549–1560.
Article30. Proctor RN. The anti-tobacco campaign of the Nazis: a little known aspect of public health in Germany, 1933-45. BMJ. 1996; 313:1450–1453.
Article31. Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014; 134:593–601.e12.32. Woodcock A, Lowe LA, Murray CS, Simpson BM, Pipis SD, Kissen P, et al. Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. Am J Respir Crit Care Med. 2004; 170:433–439.33. STREPTOMYCIN treatment of pulmonary tuberculosis. Br Med J. 1948; 2:769–782.34. Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000; 342:1887–1892.
Article35. Brass EP. The gap between clinical trials and clinical practice: the use of pragmatic clinical trials to inform regulatory decision making. Clin Pharmacol Ther. 2010; 87:351–355.
Article36. Battaglia S, Basile M, Spatafora M, Scichilone N. Are asthmatics enrolled in randomized trials representative of real-life outpatients? Respiration. 2015; 89:383–389.
Article37. Travers J, Marsh S, Williams M, Weatherall M, Caldwell B, Shirtcliffe P, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007; 62:219–223.
Article38. Krauskopf KA, Sofianou A, Goel MS, Wolf MS, Wilson EA, Martynenko ME, et al. Depressive symptoms, low adherence, and poor asthma outcomes in the elderly. J Asthma. 2013; 50:260–266.
Article39. Mosnaim G, Li H, Martin M, Richardson D, Belice PJ, Avery E, et al. Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Ann Allergy Asthma Immunol. 2014; 112:116–120.
Article40. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012; 5:CD002314.
Article41. Dorais M, Blais L, Chabot I, LeLorier J. Treatment persistence with leukotriene receptor antagonists and inhaled corticosteroids. J Asthma. 2005; 42:385–393.
Article42. Price D, Musgrave SD, Shepstone L, Hillyer EV, Sims EJ, Gilbert RF, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011; 364:1695–1707.
Article43. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005; 353:487–497.
Article44. Woodcock A, Bakerly ND, New JP, Gibson JM, Wu W, Vestbo J, et al. The Salford Lung Study protocol: a pragmatic, randomised phase III real-world effectiveness trial in asthma. BMC Pulm Med. 2015; 15:160.
Article45. Bateman ED, O'Byrne PM, Busse WW, Lotvall J, Bleecker ER, Andersen L, et al. Once-daily fluticasone furoate (FF)/vilanterol reduces risk of severe exacerbations in asthma versus FF alone. Thorax. 2014; 69:312–319.
Article46. Garbutt JM, Yan Y, Highstein G, Strunk RC. A cluster-randomized trial shows telephone peer coaching for parents reduces children's asthma morbidity. J Allergy Clin Immunol. 2015; 135:1163–1170.e1-2.
Article47. Kramer MS, Matush L, Vanilovich I, Platt R, Bogdanovich N, Sevkovskaya Z, et al. Effect of prolonged and exclusive breast feeding on risk of allergy and asthma: cluster randomised trial. BMJ. 2007; 335:815.
Article48. Campbell MK, Piaggio G, Elbourne DR, Altman DG. CONSORT Group. Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012; 345:e5661.
Article49. Castro-Rodriguez JA, Garcia-Marcos L. Wheezing and Asthma in childhood: an epidemiology approach. Allergol Immunopathol (Madr). 2008; 36:280–290.
Article50. Halterman JS, Fagnano M, Tremblay PJ, Fisher SG, Wang H, Rand C, et al. Prompting asthma intervention in Rochester-uniting parents and providers (PAIR-UP): a randomized trial. JAMA Pediatr. 2014; 168:e141983.51. Lurie JD, Morgan TS. Pros and cons of pragmatic clinical trials. J Comp Eff Res. 2013; 2:53–58.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Environmental Factors Affecting Prevalence of Allergic Diseases in Elementary School Children in a Province
- Particulate matter and childhood allergic diseases
- The Prevalence of Atopic Dermatitis, Asthma, and Allergic Rhinitis and the Comorbidity of Allergic Diseases in Children
- Impact of perinatal environmental tobacco smoke on the development of childhood allergic diseases
- Geographical and Sociodemographic Risk Factors for Allergic Diseases in Korean Children