Clin Endosc.
2016 Sep;49(5):483-487. 10.5946/ce.2016.008.
Synchronous Peripancreatic Lymph Node Gastrinoma and Gastric Neuroendocrine Tumor Type 2
- Affiliations
-
- 1Department of Internal Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea. junwonchung@hanmail.net
- 2Department of Surgery, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea.
- 3Department of Hematology, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea.
- KMID: 2356058
- DOI: http://doi.org/10.5946/ce.2016.008
Abstract
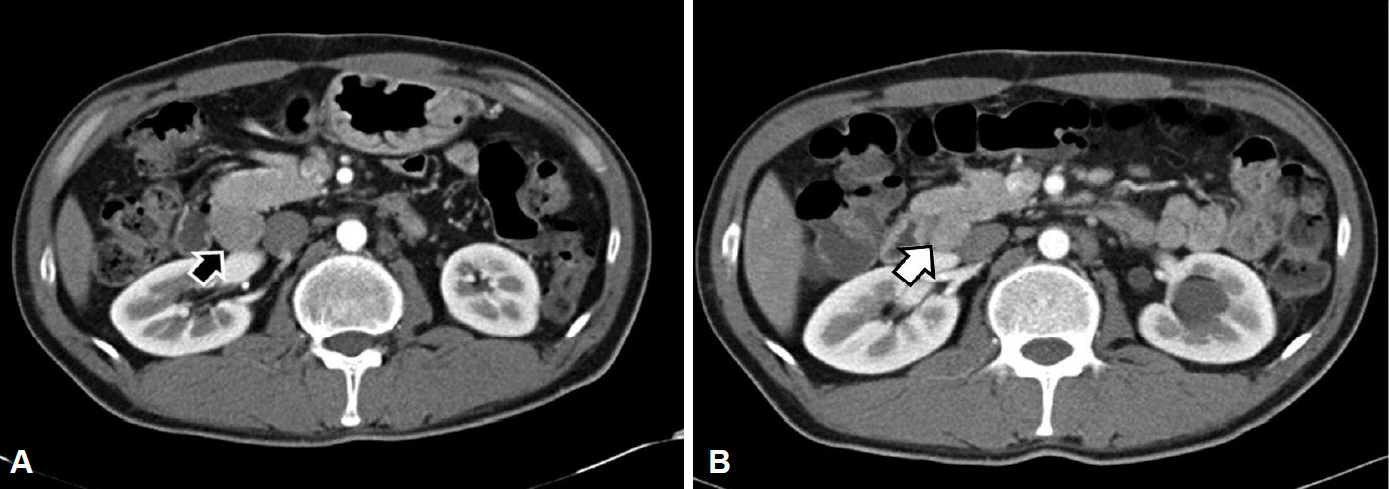
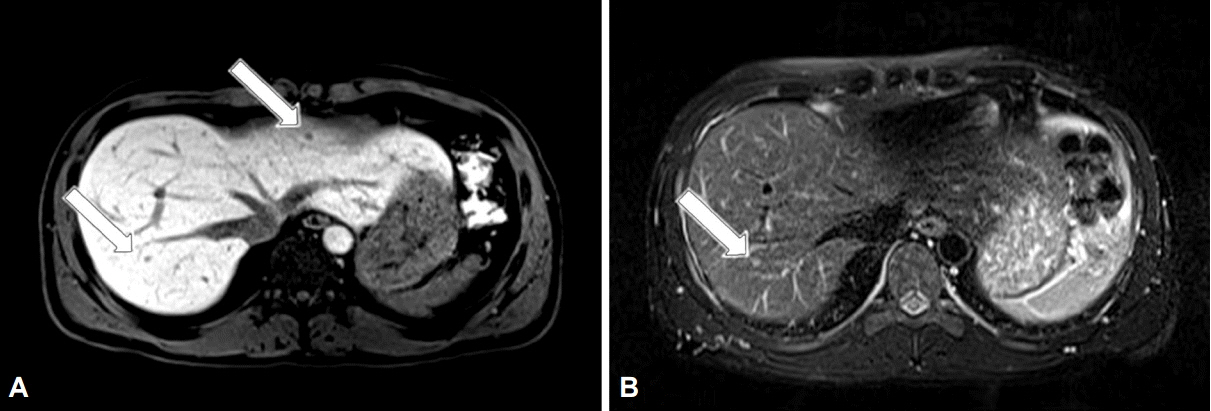
- A 34-year-old man was referred to our hospital with gastric polypoid lesions and biopsy-confirmed neuroendocrine tumor (NET). Computed tomography (CT) revealed a 3×3.5×8-cm retroperitoneal mass behind the pancreas, with multiple hepatic metastases. His serum gastrin level was elevated to 1,396 pg/mL. We performed a wedge resection of the stomach, a right hemi-hepatectomy, and a retroperitoneal mass excision. After careful review of the clinical, radiological, histopathological, and immunohistochemical findings, peripancreatic gastrinoma, and synchronous gastric NET were ultimately diagnosed. We reviewed a CT scan that had been performed 6 years previously after surgery for a duodenal perforation. There was no evidence of gastric or hepatic lesions, but the retroperitoneal mass was present at the same site. Had gastrinoma been detected earlier, our patient could have been cured using less invasive treatment. This case demonstrates how important it is to consider Zollinger-Ellison syndrome in patients with a recurrent or aggressive ulcer.
MeSH Terms
Figure
Reference
-
1. Passaro E Jr, Howard TJ, Sawicki MP, Watt PC, Stabile BE. The origin of sporadic gastrinomas within the gastrinoma triangle: a theory. Arch Surg. 1998; 133:13–16.2. Mian O, Mahmoud A, Ibrahim M, Rassai H. Primary gastrinoma of lymph node: fact or fiction? Am Surg. 2009; 75:208–211.3. Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Possible primary lymph node gastrinoma: occurrence, natural history, and predictive factors: a prospective study. Ann Surg. 2003; 237:650–657.4. Anlauf M, Enosawa T, Henopp T, et al. Primary lymph node gastrinoma or occult duodenal microgastrinoma with lymph node metastases in a MEN1 patient: the need for a systematic search for the primary tumor. Am J Surg Pathol. 2008; 32:1101–1105.5. Herrmann ME, Ciesla MC, Chejfec G, DeJong SA, Yong SL. Primary nodal gastrinomas. Arch Pathol Lab Med. 2000; 124:832–835.
Article6. Crosby DA, Donohoe CL, Fitzgerald L, et al. Gastric neuroendocrine tumours. Dig Surg. 2012; 29:331–348.7. Berna MJ, Annibale B, Marignani M, et al. A prospective study of gastric carcinoids and enterochromaffin-like cell changes in multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: identification of risk factors. J Clin Endocrinol Metab. 2008; 93:1582–1591.
Article8. Norton JA, Melcher ML, Gibril F, Jensen RT. Gastric carcinoid tumors in multiple endocrine neoplasia-1 patients with Zollinger-Ellison syndrome can be symptomatic, demonstrate aggressive growth, and require surgical treatment. Surgery. 2004; 136:1267–1274.
Article9. Jang SH, Jang SM, Jun YJ, et al. Peripancreatic lymph node gastrinoma. Basic Appl Pathol. 2009; 2:140–142.
Article10. Howard TJ, Zinner MJ, Stabile BE, Passaro E Jr. Gastrinoma excision for cure. A prospective analysis. Ann Surg. 1990; 211:9–14.11. Zhou H, Schweikert HU, Wolff M, Fischer HP. Primary peripancreatic lymph node gastrinoma in a woman with MEN1. J Hepatobiliary Pancreat Surg. 2006; 13:477–481.
Article12. Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology. 2009; 89:471–476.
Article13. Yu F, Venzon DJ, Serrano J, et al. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol. 1999; 17:615–630.
Article14. Cadiot G, Vuagnat A, Doukhan I, et al. Prognostic factors in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. Groupe d’Etude des Neoplasies Endocriniennes Multiples (GENEM and groupe de Recherche et d’Etude du Syndrome de Zollinger-Ellison (GRESZE). Gastroenterology. 1999; 116:286–293.15. Jensen RT, Niederle B, Mitry E, et al. Gastrinoma (duodenal and pancreatic). Neuroendocrinology. 2006; 84:173–182.
Article16. Bartlett EK, Roses RE, Gupta M, et al. Surgery for metastatic neuroendocrine tumors with occult primaries. J Surg Res. 2013; 184:221–227.
Article17. Capurso G, Rinzivillo M, Bettini R, Boninsegna L, Delle Fave G, Falconi M. Systematic review of resection of primary midgut carcinoid tumour in patients with unresectable liver metastases. Br J Surg. 2012; 99:1480–1486.
Article18. Givi B, Pommier SJ, Thompson AK, Diggs BS, Pommier RF. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery. 2006; 140:891–897.
Article19. Touzios JG, Kiely JM, Pitt SC, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005; 241:776–783.20. Norton JA, Kivlen M, Li M, Schneider D, Chuter T, Jensen RT. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003; 138:859–866.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metastatic Large Cell Neuroendocrine Carcinoma Combined with Gastric Adenocarcinoma
- A Case of a Gastric Composite Tumor with an Adenocarcinoma and a Large Cell Neuroendocrine Carcinoma
- Mixed Exocrine and Endocrine Carcinoma in the Stomach: A Case Report
- Gastric Adenocarcinoma with Coexistent Hepatoid Adenocarcinoma and Neuroendocrine Carcinoma: A Case Report
- A Case of Zollinger-Ellison Syndrome with Gastrinoma Localized by 111In-Pentetreotide Scan