Development of New Therapies for Severe Asthma
- Affiliations
-
- 1Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Pittsburgh Asthma Institute at UPMC/University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA. wenzelse@upmc.edu
- KMID: 2355889
- DOI: http://doi.org/10.4168/aair.2017.9.1.3
Abstract
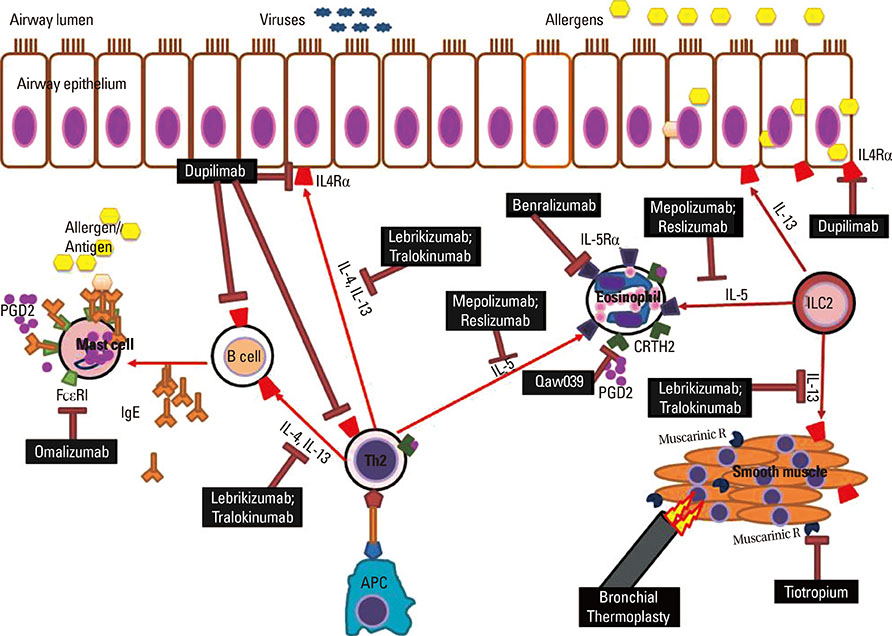
- Persistent asthma has long been treated with inhaled corticosteroids (CSs), as the mainstay of therapy. However, their efficacy in patients with more severe disease is limited, which led to the incorporation of poor response to ICSs (and thereby use of high doses of ICS) into recent definitions of severe asthma. Several studies have suggested that severe asthma might consist of several different phenotypes, each with ongoing symptoms and health care utilization, despite the use of high doses of ICS, usually in combination with a second or third controller. Several new therapies have been approved for severe asthma. Long-acting muscarinic agents have recently been approved as an additional controller agent and appear to improve lung function, although their effect on symptoms and exacerbations is less. Although bronchial thermoplasty (BT) has emerged as a therapy for severe asthma, little is understood regarding the appropriate selection of these patients. Considerable data have emerged to support the presence of a group of patients with severe asthma who have ongoing Type 2 inflammation. These patients appear to respond to targeted biologic approaches which are at the current time mostly investigational. In contrast, few effective therapies for patients with less or no evidence for Type 2 inflammation have emerged. Many new and exciting therapies are at the forefront for severe asthma therapy and, in conjunction with precision medicine approaches to identify the group of patients likely to respond to these approaches, will change the way we think about treating severe asthma.
MeSH Terms
Figure
Cited by 11 articles
-
TRPV1 Blocking Alleviates Airway Inflammation and Remodeling in a Chronic Asthma Murine Model
Joon Young Choi, Hwa Young Lee, Jung Hur, Kyung Hoon Kim, Ji Young Kang, Chin Kook Rhee, Sook Young Lee
Allergy Asthma Immunol Res. 2018;10(3):216-224. doi: 10.4168/aair.2018.10.3.216.Lung Function Trajectory Types in Never-Smoking Adults With Asthma: Clinical Features and Inflammatory Patterns
Joo-Hee Kim, Hun Soo Chang, Seung Woo Shin, Dong Gyu Baek, Ji-Hye Son, Choon-Sik Park, Jong-Sook Park
Allergy Asthma Immunol Res. 2018;10(6):614-627. doi: 10.4168/aair.2018.10.6.614.Is Omalizumab a Problem-Solving Remedy in Severe Asthma?
Doh Hyung Kim, Young-Koo Jee
Allergy Asthma Immunol Res. 2018;10(2):95-96. doi: 10.4168/aair.2018.10.2.95.Which Factors Associated With Activated Eosinophils Contribute to the Pathogenesis of Aspirin-Exacerbated Respiratory Disease?
Youngwoo Choi, Youngsoo Lee, Hae-Sim Park
Allergy Asthma Immunol Res. 2019;11(3):320-329. doi: 10.4168/aair.2019.11.3.320.Evaluation of Neutrophil Activation Status According to the Phenotypes of Adult Asthma
Seung-Hyun Kim, Udval Uuganbayar, Hoang Kim Tu Trinh, Duy Le Pham, Namhyo Kim, Minji Kim, Hyeukjun Sohn, Hae-Sim Park
Allergy Asthma Immunol Res. 2019;11(3):381-393. doi: 10.4168/aair.2019.11.3.381.Characteristics of Adult Severe Refractory Asthma in Korea Analyzed From the Severe Asthma Registry
Min-Hye Kim, Sang-Heon Kim, So-Young Park, Ga-Young Ban, Joo-Hee Kim, Jae-Woo Jung, Ji Yong Moon, Woo-Jung Song, Hyouk-Soo Kwon, Jae-Woo Kwon, Jae Hyun Lee, Hye-Ryun Kang, Jong-Sook Park, Tae-Bum Kim, Heung-Woo Park, Kwang-Ha Yoo, Yeon-Mok Oh, Young-Il Koh, An-Soo Jang, Byung-Jae Lee, Young-Joo Cho, Sang-Heon Cho, Hae-Sim Park, Choon-Sik Park, Ho Joo Yoon, You Sook Cho
Allergy Asthma Immunol Res. 2019;11(1):43-54. doi: 10.4168/aair.2019.11.1.43.Future Risks in Patients With Severe Asthma
Woo-Jung Song, Ji-Hyang Lee, Yewon Kang, Woo Joung Joung, Kian Fan Chung
Allergy Asthma Immunol Res. 2019;11(6):763-778. doi: 10.4168/aair.2019.11.6.763.Therapeutic Effect of Omalizumab in Severe Asthma: A Real-World Study in Korea
Ji-Ho Lee, Hyun Young Lee, Chang-Gyu Jung, Ga-Young Ban, Yoo Seob Shin, Young-Min Ye, Dong-Ho Nahm, Hae-Sim Park
Allergy Asthma Immunol Res. 2018;10(2):121-130. doi: 10.4168/aair.2018.10.2.121.Perceptions of Severe Asthma and Asthma-COPD Overlap Syndrome Among Specialists: A Questionnaire Survey
Sang-Heon Kim, Ji Yong Moon, Jae Hyun Lee, Ga-Young Ban, Sujeong Kim, Mi-Ae Kim, Joo-Hee Kim, Min-Hye Kim, Chan-Sun Park, So-Young Park, Hyouk-Soo Kwon, Jae-Woo Kwon, Jae-Woo Jung, Hye-Ryun Kang, Jong-Sook Park, Tae-Bum Kim, Heung-Woo Park, You Sook Cho, Kwang-Ha Yoo, Yeon-Mok Oh, Byung-Jae Lee, An-Soo Jang, Sang-Heon Cho, Hae-Sim Park, Choon-Sik Park, Ho Joo Yoon,
Allergy Asthma Immunol Res. 2018;10(3):225-235. doi: 10.4168/aair.2018.10.3.225.An Alternative Dendritic Cell-Induced Murine Model of Asthma Exhibiting a Robust Th2/Th17-Skewed Response
Sang Chul Park, Hongmin Kim, Yeeun Bak, Dahee Shim, Kee Woong Kwon, Chang-Hoon Kim, Joo-Heon Yoon, Sung Jae Shin
Allergy Asthma Immunol Res. 2020;12(3):537-555. doi: 10.4168/aair.2020.12.3.537.Successful additional clarithromycin and tacrolimus treatment for hypereosinophilia associated with eosinophilic granulomatosis with polyangiitis
Masashi Ohe, Haruki Shida, Tetsuya Horita, Ken Furuya
Transl Clin Pharmacol. 2018;26(2):60-63. doi: 10.12793/tcp.2018.26.2.60.
Reference
-
1. National Heart, Lung, and Blood Institute (US). Guidelines for the diagnosis and management of asthma: expert panel report 3 [Internet]. Bethesda (MD): U.S. Department of Health and Human Services;2007. cited 2015 Nov 11. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.2. Chung KF, Wenzel S. European Respiratory Society/American Thoracic Society Severe Asthma International Guidelines Task Force. From the authors: International European Respiratory Society/American Thoracic Society guidelines on severe asthma. Eur Respir J. 2014; 44:1378–1379.3. Wenzel SE, Busse WW. National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Severe asthma: lessons from the Severe Asthma Research Program. J Allergy Clin Immunol. 2007; 119:14–21.4. Brown HM. Treatment of chronic asthma with prednisolone; significance of eosinophils in the sputum. Lancet. 1958; 2:1245–1247.5. Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999; 353:2213–2214.6. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009; 360:973–984.7. Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009; 360:985–993.8. Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007; 104:15858–15863.9. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009; 180:388–395.10. Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012; 130:647–654.11. Corren J, Lemanske RF Jr, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–1098.12. Chibana K, Trudeau JB, Mustovich AT, Hu H, Zhao J, Balzar S, et al. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy. 2008; 38:936–946.13. Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SA, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010; 181:1033–1041.14. Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007; 370:1422–1431.15. Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013; 368:2455–2466.16. Kerstjens HA, Disse B, Schröder-Babo W, Bantje TA, Gahlemann M, Sigmund R, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011; 128:308–314.17. Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012; 367:1198–1207.18. Boehringer Ingelheim GmbH (DE). Asthma: U.S. FDA approves new indication for SPIRIVA® Respimat® [Internet]. cited 2015 Nov 11. Avaliable from: http://us.boehringer-ingelheim.com/news_events/press_releases/press_release_archive/2015/fda-approves-boehringer-ingelheims-spiriva-respimat-maintenance-treatment-asthma-adults-adolescents.html.19. Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet]. Global Initiative for Asthma;2015. updated 2015 Apr. acessed 2015 Oct 17. Available from: http://www.ginasthma.org/local/uploads/files/GINA_Report_2015.pdf.20. Evans DJ, Kew KM, Anderson DE, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus higher dose ICS for adults with asthma. Cochrane Database Syst Rev. 2015; CD011437.21. Anderson DE, Kew KM, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus the same dose of ICS alone for adults with asthma. Cochrane Database Syst Rev. 2015; CD011397.22. Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010; 181:116–124.23. Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol. 2011; 107:65–70.24. Wechsler ME, Laviolette M, Rubin AS, Fiterman J, Lapa e Silva JR, Shah PL, et al. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013; 132:1295–1302.25. Pretolani M, Dombret MC, Thabut G, Knap D, Hamidi F, Debray MP, et al. Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med. 2014; 190:1452–1454.26. Chakir J, Haj-Salem I, Gras D, Joubert P, Beaudoin ÈL, Biardel S, et al. Effects of bronchial thermoplasty on airway smooth muscle and collagen deposition in asthma. Ann Am Thorac Soc. 2015; 12:1612–1618.27. Denner DR, Doeing DC, Hogarth DK, Dugan K, Naureckas ET, White SR. Airway inflammation after bronchial thermoplasty for severe asthma. Ann Am Thorac Soc. 2015; 12:1302–1309.28. Torrego A, Solà I, Munoz AM, Roqué I, Yepes-Nuñez JJ, Alonso-Coello P, et al. Bronchial thermoplasty for moderate or severe persistent asthma in adults. Cochrane Database Syst Rev. 2014; CD009910.29. Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001; 108:184–190.30. Solèr M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001; 18:254–261.31. Holgate ST, Chuchalin AG, Hébert J, Lötvall J, Persson GB, Chung KF, et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy. 2004; 34:632–638.32. Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011; 154:573–582.33. Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013; 187:804–811.34. Walker S, Monteil M, Phelan K, Lasserson TJ, Walters EH. Anti-IgE for chronic asthma in adults and children. Cochrane Database Syst Rev. 2006; CD003559.35. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014; CD003559.36. Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004; 113:101–108.37. Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008; 178:218–224.38. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010; 181:315–323.39. Amelink M, de Groot JC, de Nijs SB, Lutter R, Zwinderman AH, Sterk PJ, et al. Severe adult-onset asthma: a distinct phenotype. J Allergy Clin Immunol. 2013; 132:336–341.40. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000; 162:2341–2351.41. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 380:651–659.42. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014; 371:1198–1207.43. Prazma CM, Wenzel S, Barnes N, Douglass JA, Hartley BF, Ortega H. Characterisation of an OCS-dependent severe asthma population treated with mepolizumab. Thorax. 2014; 69:1141–1142.44. Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014; 371:1189–1197.45. Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011; 184:1125–1132.46. Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015; 3:355–366.47. Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010; 125:1344–1353.e2.48. Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014; 2:879–890.49. Nowak RM, Parker JM, Silverman RA, Rowe BH, Smithline H, Khan F, et al. A randomized trial of benralizumab, an antiinterleukin 5 receptor α monoclonal antibody, after acute asthma. Am J Emerg Med. 2015; 33:14–20.50. Katz LE, Gleich GJ, Hartley BF, Yancey SW, Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014; 11:531–536.51. Nucala (mepolizumab): highlights of prescribing information [Internet]. Philadelphia (PA): GlaxoSmithKline LLC;2015. 11. cited 2015 Dec 1. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL.PDF.52. Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010; 181:788–796.53. Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J. 2013; 41:330–338.54. Brightling CE, Chanez P, Leigh R, O'Byrne PM, Korn S, She D, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015; 3:692–701.55. Hanania NA, Noonan M, Corren J, Korenblat P, Zheng Y, Fischer SK, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015; 70:748–756.56. De Boever EH, Ashman C, Cahn AP, Locantore NW, Overend P, Pouliquen IJ, et al. Efficacy and safety of an anti-IL-13 mAb in patients with severe asthma: a randomized trial. J Allergy Clin Immunol. 2014; 133:989–996.57. Wenzel SE, Wang L, Pirozzi G, Sutherland ER, Graham N, Evans RR, et al. Dupilumab improves lung function and reduces severe exacerbations in uncontrolled asthmatics with baseline eosinophil levels above and below 300 cells/μL. Am J Respir Crit Care Med. 2015; 191:A6362.58. Balzar S, Fajt ML, Comhair SA, Erzurum SC, Bleecker E, Busse WW, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am J Respir Crit Care Med. 2011; 183:299–309.59. Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D2 pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013; 131:1504–1512.60. Pettipher R, Hansel TT, Armer R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat Rev Drug Discov. 2007; 6:313–325.61. Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013; 14:536–542.62. Barnes N, Pavord I, Chuchalin A, Bell J, Hunter M, Lewis T, et al. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin Exp Allergy. 2012; 42:38–48.63. Pettipher R, Hunter MG, Perkins CM, Collins LP, Lewis T, Baillet M, et al. Heightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459. Allergy. 2014; 69:1223–1232.64. Hall IP, Fowler AV, Gupta A, Tetzlaff K, Nivens MC, Sarno M, et al. Efficacy of BI 671800, an oral CRTH2 antagonist, in poorly controlled asthma as sole controller and in the presence of inhaled corticosteroid treatment. Pulm Pharmacol Ther. 2015; 32:37–44.65. Berair R, Singapuri A, Hartley R, Laurencin M, Bacher G, Holzhauer B, et al. Effect of Qaw039, an oral prostaglandin D2 receptor (DP2/CrTh2) antagonist, upon sputum and bronchial eosinophilic inflammation and clinical outcomes in treatment-resistant asthma: a phase 2a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2015; 191:A6361.66. Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006; 354:697–708.67. Morjaria JB, Chauhan AJ, Babu KS, Polosa R, Davies DE, Holgate ST. The role of a soluble TNFalpha receptor fusion protein (etanercept) in corticosteroid refractory asthma: a double blind, randomised, placebo controlled trial. Thorax. 2008; 63:584–591.68. Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlén SE, et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009; 179:549–558.69. Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010; 115:335–343.70. Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013; 188:1294–1302.71. Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015; 386:1086–1096.72. Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997; 156:737–743.73. Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999; 160:1532–1539.74. Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010; 125:1028–1036.e13.75. Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014; 133:1557–1563.76. Nair P, Gaga M, Zervas E, Alagha K, Hargreave FE, O'Byrne PM, et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2012; 42:1097–1103.77. Uzun S, Djamin RS, Kluytmans JA, Mulder PG, van't Veer NE, Ermens AA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014; 2:361–368.78. Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EH, Koppers RJ, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013; 309:1251–1259.79. Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, Brain B, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA. 2013; 309:1260–1267.80. Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013; 68:322–329.81. Mullarkey MF, Lammert JK, Blumenstein BA. Long-term methotrexate treatment in corticosteroid-dependent asthma. Ann Intern Med. 1990; 112:577–581.82. Erzurum SC, Leff JA, Cochran JE, Ackerson LM, Szefler SJ, Martin RJ, et al. Lack of benefit of methotrexate in severe, steroid-dependent asthma. A double-blind, placebo-controlled study. Ann Intern Med. 1991; 114:353–360.83. Davies H, Olson L, Gibson P. Methotrexate as a steroid sparing agent for asthma in adults. Cochrane Database Syst Rev. 2000; CD000391.84. Wenzel SE, Vitari CA, Shende M, Strollo DC, Larkin A, Yousem SA. Asthmatic granulomatosis: a novel disease with asthmatic and granulomatous features. Am J Respir Crit Care Med. 2012; 186:501–507.